
New study from a collaborative team between Dr. Ross Howie of HPSTAR, Prof. Eugene Gregoryanz of HPSTAR and SHARPS and Dr. Miriam Pena-Alvarez, of University of Edinburgh has revised the phase diagram of methane -- a textbook example of a chemical compound that is important in fundamental and environmental sciences as well as in industry. The results were published in Physical Review Letters and featured in Physics as Editors’ suggestion.
Because of the deceptive simplicity of its molecule and being the main constituent of natural gas found on Earth methane was arguably studied more than some other iconic compounds or archetypal elements. Surprisingly, in the literature there are still some discrepancies and controversies in the phase diagram of this common gas at high temperatures (above 300 K).
By conducting dozens of in situ high-pressure and high-temperature Raman spectroscopy experiments, the team conducted the systematic exploration of the phase diagram resolving inconsistencies of earlier studies.
The experiments yielded two distinct phase diagrams, one that demonstrates the kinetic phase transformations and the other presenting the equilibrium states usually reached with time. Raman spectroscopy demonstrated that the appearance and transitions between the higher pressure phases (VII, VIII, and IX) are strongly dependent on the pressure-temperature-time path. By combining the visual observations and optical spectroscopy the authors were able to suggest that the melting curve of methane extends to significantly higher temperatures than previously reported, e.g., ∼1000 K at 15 GPa. The first author of the study, the PhD student of Prof. Gregoryanz at U of Edinburgh and earlier Master student of Dr. Howie at HPSTAR, Dr Wang Mengnan said: the high pressure studies of methane require a lot patience and attention – we were waiting for days and sometimes for months to reach the equilibrium state and we found out that some of the inconsistencies in the earlier melting data could be attributed to photochemical dissociation and/or a reaction induced by high-intensity light sources, the fact which was often missed in the earlier studies.
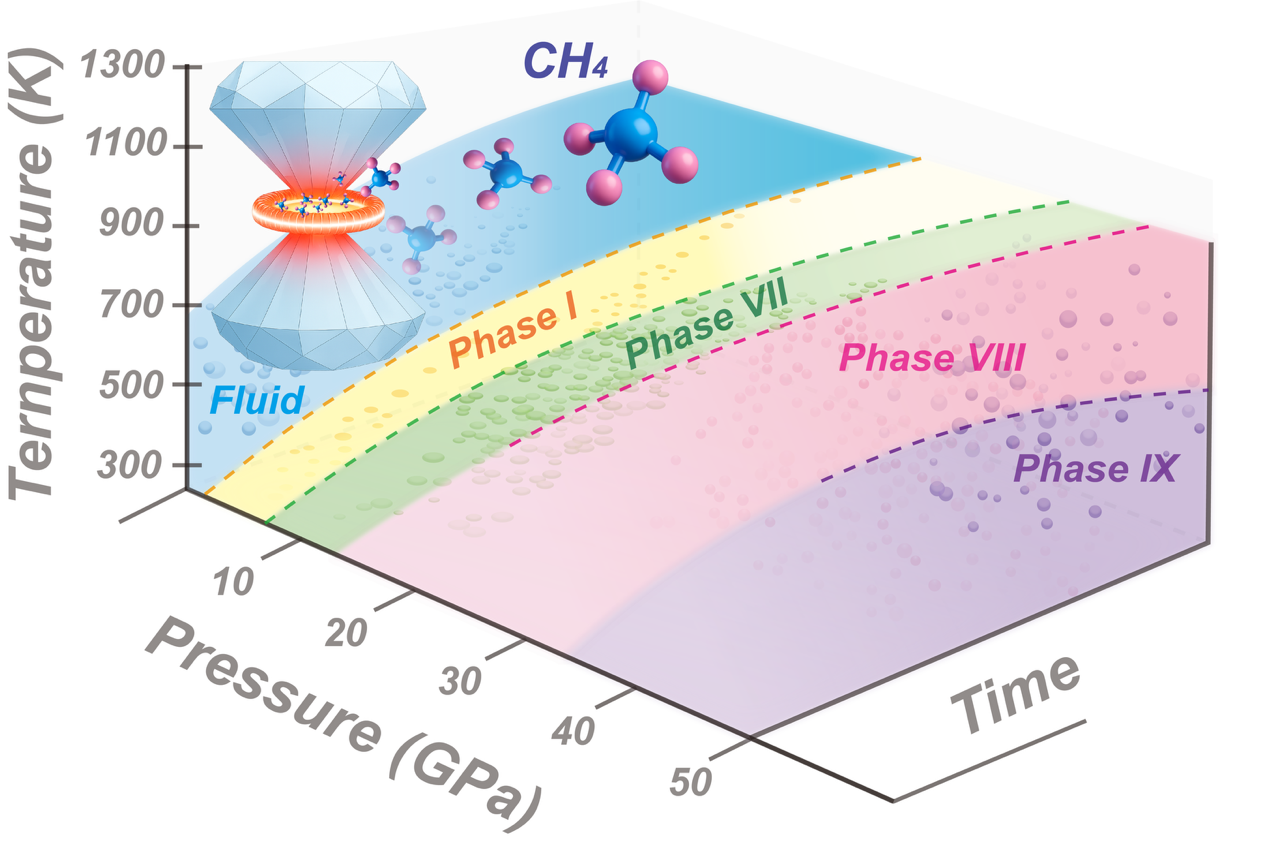
甲烷分子结构看似简单,且作为地球天然气的主要成分,其受研究程度可以说远超部分标志性化合物或典型元素。令人意外的是,这种常见气体在高温条件下(300K 以上)的相图仍存在若干分歧与争议。该研究团队通过开展数十组原位高温高压拉曼光谱实验,对甲烷相图进行了系统性探究,厘清了早期研究中存在的矛盾之处。实验最终得到两类截然不同的相图:一类反映动力学相变过程,另一类则呈现体系经充分弛豫后达到的平衡态。拉曼光谱测试结果表明,甲烷高压相(VII、VIII、IX 相)的形成及相间转变,与压力 - 温度 - 时间路径密切相关。