
Hexagonal diamond was predicted six decades ago, and subsequently synthesized by dynamic explosion and static compression. Natural hexagonal diamond was first discovered in Canyon Diablo iron meteorite, and named lonsdaleite after the pioneer women crystallgrapher Kathleen Lonsdale. As the sister allotrope of cubic diamond, hexagonal diamond holds many superior mechanical properties than cubic diamond from theoretical predictions. But previously known synthetic or natural hexagonal diamond samples are fragile, fine-grain mixtures of hexagonal diamond with various proportions of cubic diamonds, amorphous carbon or residual graphite. It is crucial to find a pathway to synthesize a pure and bulk hexagonal diamond, and confirm its properties. Recently, a research team led by Drs. Ho-kwang Mao, Liuxiang Yang and Wenge Yang from HPSTAR made a breakthrough in successful synthesis of bulk hexagonal diamond under careful control process with pressure and temperature. By utilizing a suite of advanced synchrotron and electron microscopy techniques, they confirmed the synthesized sample is pure, bulk (100 micrometer to millimeter size) hexagonal diamond phase with 100% sp³ bonding character. Comparing to the single bond length in cubic diamand (1.54 Å), hexagonal diamond owns two bond lengths which largely enhances the strength between hexagonal layers, which offers some new fantastic properties different with cubic diamond. This finding has been recently published in NATURE.
After many attempts and explorations on the synthesis of hexagonal diamond over last eight years, the HPSTAR research team solved all key issues of the graphite-hexagonal diamond transition. Starting with a high quality single crystal graphite, they conducted the in-situ synchrotron x-ray diffraction to watch the evolution of single crystal diffraction with well controlled pressure step and hydrostatic pressure-transmitting medium in a diamond anvil cell. For the first time, the hexagonal diamond crystal has been witnessed to grow epitaxially from graphite substrate, and upon laser heating at high pressure, the bulk, pure hexagonal diamond sample was successfully quenched to ambient pressure, similar to the high P-T synthesis of cubic diamond. A suite of synchrotron and atomistic transmission electron microscopy diagonostic probes has been utilized to confirm the pure sp3 bonding feature, high quality pure and bulk hexagonal diamond crystal. The highly long-range ordered, single-crystal like hexagonal diamond is fundamentally distinctive from the disordered nano-precipitation or dense planar defects in cubic diamond matrix from previous reports. More strikingly, based on the bulk averaged Raman spectroscopy and direct observation of atomistic TEM study, two bond lengths were confirmed with a regular 1.58 Å intralayer bond and a shortened 1.50 Å interlayer bond that strengthens the linkage between layer relative to the cubic diamond structure. These specific features of hexagoanl diamond are sure to afford some new fantastic properties different with cubic diamond, which opens a new direction for us to achieve more novel sp3 carbon allotropes with excellent properties.
Our results unambiguously demonstrate the existence of hexagonal diamond as a bona fide phase of carbon with superior hardness alike cubic diamond. Very excitingly, this is just the beginning; hexagonal diamond as a hexagonal variant of the cubic diamond has been predicted with many extremely favorable mechanical, electrical, thermal and optical properties comparable and complementary to diamond. Hexagonal diamond presents great opportunities for exploration in the P-T conditions of synthesis, dopants in the precursor graphite, and nano-crystallinity to optimize the desirable specific features and to engineer the ideal sp3 carbon allotrope.
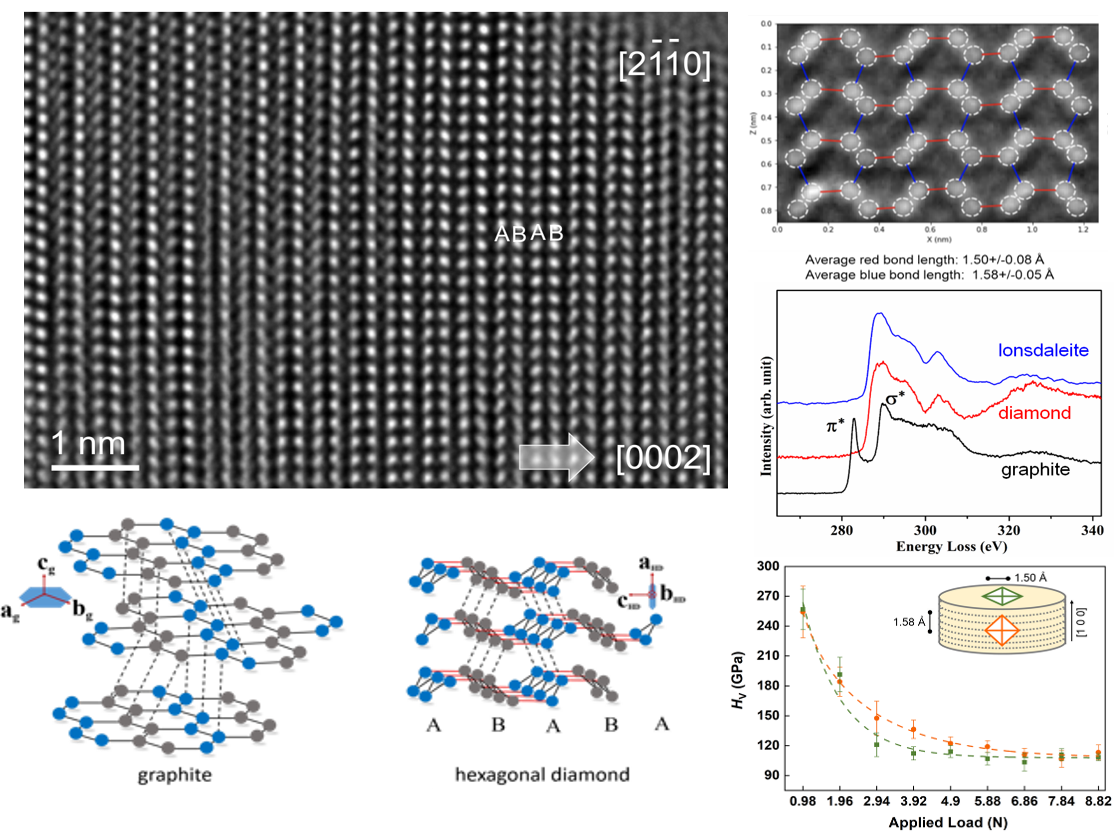
Caption: (Upper left)Atomistic view of a typical large size uniform hexagonal diamond along the [2 -1 -1 0] axis. The clear ABAB stacking order present a well arranged 2H structure. (Lower left) The epitaxially grown relationship and transition pathway from graphite to hexagonal diamond. (Right panel) Stastical counting of shortened interlayer bond (red) and regular intralayer bond (blue), EELS spectra from three carbon allotropes, and the Vicker hardness measurement from the millimeter sized bulk hexagonal diamond synthesized in a large volume press.
Media reports: CHEMISTRY WORLD: Rare diamond with unique hexagonal structure is harder than natural counterpart
新华网:https://english.news.cn/20250731/ea81bd8ee9984571801a4b4b2603fbc6/c.html
在人们的普遍认知中,金刚石(即钻石)特指具有立方晶体结构的sp³型碳单质。然而鲜为人知的是,sp³碳家族中还存在着一种结构奇特的六方金刚石。作为碳元素sp³杂化的另一种同素异形体,理论研究表明,六方金刚石的机械性能将远超越传统立方金刚石,使其成为材料科学界竞相追逐的圣杯级超硬材料。遗憾的是,经过六十年的实验研究,高纯度六方金刚石的合成仍面临重大技术瓶颈,导致其以硬度为代表的关键性能参数始终难以通过实验来充分验证。近日,北京高压科学研究中心的杨留响研究员,杨文革研究员,以及毛河光院士与中国科学院西安光机所罗端研究员带领的国际研究团队成功制备出百微米至毫米级的高纯度类单晶块体样品。该研究表明,与仅具有单一碳-碳键长的立方金刚石不同,六方金刚石呈现出两种不同的键长分布,且层间距显著缩短,这种独特的碳原子堆垛方式,将有效克服立方金刚石的密堆积面易滑移的弱点。相关结果以《Synthesis of bulk hexagonal diamond》为题发表于Nature杂志。