Enhanced reactivity of lithium and copper at high pressure - Dr. Jack Binns
JUNE 6, 2018
High pressure can profoundly affect electronic structure and reactivity, creating compounds between elements that do not react at ambient conditions. Lithium is known to react with gold and silver, however no copper compounds are known to date. New study by a group of HPSTAR scientists reports that by compressing mixtures of the elements in diamond-anvil cells, compounds of lithium and copper have been synthesized for the first time. This study ispublished in the Journalof Physical Chemistry Letters.
Compounds of lithium-gold and lithium-silver have been known for over 50 years, however lithium-copper compounds have never been discovered despite many investigations.
“Copper comes from the same group of gold and silver, they should share similar chemical properties,” said Dr. Jack Binns, the lead author of the study. “We think that it may require some external drive to make the reaction happen”.
Here a group of HPSTAR scientists including Jack Binns, Mengnan Wang, Eugene Gregoryanz, and Ross T. Howie use pressure to modulate the reactivity between lithium and copper.
At pressure even below 1 GPa (10000 atmospheres), the team observed the reaction between lithium and copper by insitu synchrotron X-raydiffraction. The reaction product LiCu, displaying two-dimensional layers of Cu atoms, and is the first known example of the complex “mu-phase” intermetallic structure formed by an alkali metal.
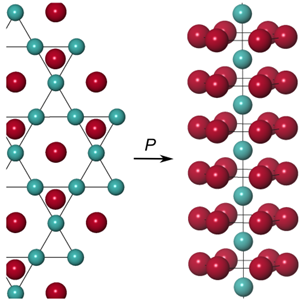
Furthermore, when they increase the pressure above 5 GPa, the layered bonding is replaced by linear chains of Cu atoms in the high-pressure phase Li2Cu.
“Our study provides the first experimental evidence of lithium-copper compounds”, said Dr. Ross Howie, the corresponding author. Lithium is an important element in energy storage technology and our findings show high-pressure methods are auseful way to modify its reactivity”.
Synthesizing these compounds has important implications for high-pressure resistivity experiments utilizing Cu as electrodes. From this study, even at pressures created during sample loading they form Li-Cu compounds that persist to at least 25 GPa. So the recent high-pressure resistivity studies on lithium using copper as electrodes would definitely contain significant contamination.
Caption:At low pressuresLiCu contains hexagonal layers of Cu atoms which transform with increasing pressure into linear chains.
锂作为元素周期表中密度最小的金属,其化学性质十分活泼,能与元素周期表中的大部分金属,非金属反应,甚至能与金,银这些惰性金属反应生成合金。然而作为与金,银同一主族的铜元素,至今未有锂铜合金的发现。北京高压科学研究中心的Howie团队使用高压的手段来调节锂-铜之间的化学反应。在金刚石压砧中封装样品的条件下(<1 GPa),该研究团队通过同步辐射X射线衍射观察到了理-铜(LiCu)化合物的形成。在更高的压力(>5 GPa)时又发现了 Li2Cu的形成。该发现是锂-铜化合物的第一个实验证据,对锂在能源存储方面的应用将有一定的指导意义。另外该研究指出既然锂,铜会在如此低的压力下反应,那么就不能使用铜作为电极材料来测量锂的电学性质。
