A degassing mechanism for deep hydrous mineral - Dr. Qingyang Hu
AUGUST 23, 2017
Water (H2O) is one of the most common molecules on Earth and an indispensable ingredient for the existence of life. At ambient pressure, water is a transparent liquid that is generally stable between the freezing point (0 °C) and the boiling point (100 °C). By applying external pressure to a few thousands of atmospheres, water turns into ice even at room temperature. Underneath the earth’s surface where the pressure and temperature is very high, most water molecules will be associated with rocks to form the so-called hydrous minerals. During the deep subduction containing hydrous minerals, a majority of them will dehydrate due to climbing temperature at the bottom of Earth lower mantle such that it completes its cycling in deep Earth.
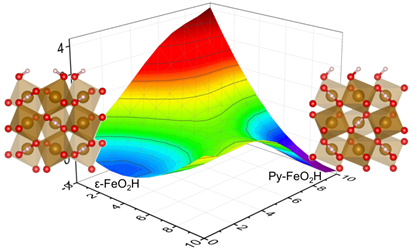 Thanks to the symmetric O-H-O bonding, a certain class of hydrous minerals is recently discovered to survive in the high-pressure high-temperature condition that mimics the deep lower mantle. The composition FeO2H, named goethite when they are found on Earth surface, is one of such hydrous minerals. It forms symmetric O-H-O framework at high-pressure hence features higher dehydration temperature. However, at even deeper depth scientists suggest new profound chemistry related to FeO2H. New works from a team including HPSTAR’s Shengcai Zhu, Qingyang Hu, Ho-kwang Mao, Hongwei Sheng and collaborator Wendy Mao from Stanford University has identified that such symmetric network will break above around 750,000 atmosphere and 1400 °C. Their results were published in the Journal of American Chemical Society (DOI: 10.1021/jacs.7b06528).
Thanks to the symmetric O-H-O bonding, a certain class of hydrous minerals is recently discovered to survive in the high-pressure high-temperature condition that mimics the deep lower mantle. The composition FeO2H, named goethite when they are found on Earth surface, is one of such hydrous minerals. It forms symmetric O-H-O framework at high-pressure hence features higher dehydration temperature. However, at even deeper depth scientists suggest new profound chemistry related to FeO2H. New works from a team including HPSTAR’s Shengcai Zhu, Qingyang Hu, Ho-kwang Mao, Hongwei Sheng and collaborator Wendy Mao from Stanford University has identified that such symmetric network will break above around 750,000 atmosphere and 1400 °C. Their results were published in the Journal of American Chemical Society (DOI: 10.1021/jacs.7b06528).
“Due to the breakdown of symmetric hydrogen bond, hydrogen atoms will be liberated from water” said Qingyang Hu, whose team conducted computational simulation and suggests during the structural transition to its high-pressure form (py-FeO2H), half of the O-H bonds in the mineral rupture. The theoretical simulation also supports our experiments that ~50% H are released. “This is also the first time that we address degassing at the atomic scale” Added Sheng-cai Zhu, the leading author in this work.
The dehydrogenation of FeO2H sheds light on Earth’s hydrogen cycling, which will increase margins of mantle geochemistry for realizing novel reactions that introduces a new factor in Earth’s mantle.
Caption: Free energy profile from e-FeO2H to py-FeO2H.
氢的循环在地球化学中扮演着非常重要的角色。高压的条件下,非对称的O-H···O键趋于对称化,即H原子处于两个O的中心位置。对称化后的O-H-O可以提高含水矿物的热稳定性,因此含水矿物可以深入下地幔而不发生分解。然而,H是如何在地幔深部循环的,目前尚不清楚。我们的工作结合第一性原理计算和高温高压实验,来揭示一种代表性的含水矿物,即FeO2H在深地幔的脱氢机理。实验发现大约有50%的H从ε-FeO2H到Pyrite相的转变过程中释放出来;通过新的自由能抽样算法,在最低能垒通道中,我们的计算认为有一半的O-H-O键在ε-FeO2H到更高压Py-FeO2H相的相变的中断裂,因此导致了O-H-O的对称性破坏,致使大于一般的H释放。本研究将提高我们对具有对称O-H-O结构的含水矿物在相变过程中热力学稳定性的认识,同时也提供了一种含水矿物在深层地幔下具有普遍性的脱氢机理。
