New form of carbon that's hard as a rock, yet elastic, like rubber - Drs. Jinfu Shu, and Ho-Kwang “Dave” Mao
JUNE 9, 2017
Carbon is an element of seemingly infinite possibilities. This is because the configuration of its electrons allows for numerous self-bonding combinations that give rise to a range of materials with varying properties. For example, transparent, superhard diamonds, and opaque graphite, which is used for both pencils and industrial lubricant, are comprised solely of carbon. A international team including HPSTAR scientists Drs. Jinfu Shu, Ho-kwang “Dave” Mao, have developed a form of ultrastrong, lightweight carbon that is also elastic and electrically conductive. A material with such a unique combination of properties could serve a wide variety of applications from aerospace engineering to military armor.
The scientists pressurized and heated a structurally disordered form of carbon called glassy carbon. The glassy carbon starting material was brought to about 250,000 times normal atmospheric pressure and heated to approximately 1,800 degrees Fahrenheit to create the new strong and elastic carbon. Their findings are published by Science Advances.
Scientists had previously tried subjecting glassy carbon to high pressures at both room temperature (referred to as cold compression) and extremely high temperatures. But the so-called cold-synthesized material could not maintain its structure when brought back to ambient pressure, and under the extremely hot conditions, nanocrystalline diamonds were formed.
The newly created carbon is comprised of both graphite-like and diamond-like bonding motifs, which gives rise to the unique combination of properties. Under the high-pressure synthesis conditions, disordered layers within the glassy carbon buckle, merge, and connect in various ways. This process creates an overall structure that lacks a long-range spatial order, but has a short-range spatial organization on the nanometer scale.
“Light materials with high strength and robust elasticity like this are very desirable for applications where weight savings are of the utmost importance, even more than material cost,” explained Zhisheng Zhao, one of the principal investigators on this project and also a professor of Yanshan University. “What’s more, we believe that this synthesis method could be honed to create other extraordinary forms of carbon and entirely different classes of materials.”
The other members of the team are: Meng Hu, Julong He, Wentao Hu, Dongli Yu, Hao Sun, Lingyu Liu, Zihe Li, Mengdong Ma, Jian Yu Huang, Zhongyuan Liu, Bo Xu, Yongjun Tian of the State Key Laboratory of Metastable Materials Science and Technology; Yanbin Wang of the University of Chicago; and Stephen J. Juhl of Penn State University.
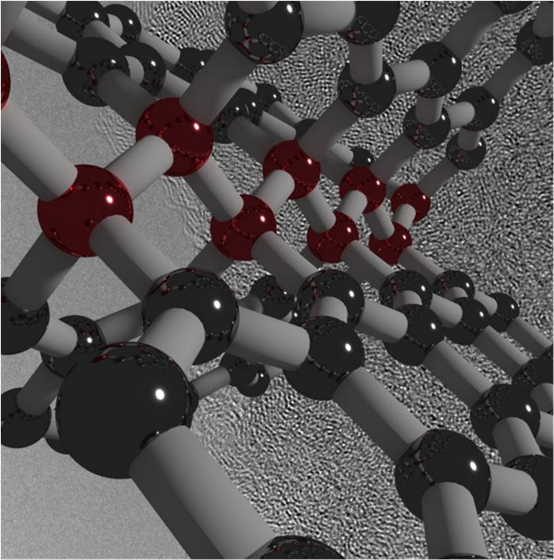
Caption: Visualization of ultrastrong, hard and elastic compressed glassy carbon. The illustrated structure is overlaid on an electron microscope image of the material. Images are provided courtesy of Timothy Strobel.
Adapted from Carnegie Institution for Science, USA.
碳具有多种同素异形体,如石墨、金刚石(钻石)、富勒烯、碳纳米管、石墨烯、玻璃碳等,其中石墨在压力下可以转变成超硬的金刚石。在此新研究中,科学家们以玻璃碳为原料,利用高压但比较温和的温度条件合成了新型碳同素异形体。它由玻璃碳压缩获得并保留了玻璃碳的一些特征,研究人员将其命名为“压缩玻璃碳”。在以前的玻璃碳在室温或高温下的高压转变研究中,制成的碳材料在卸压后又变回了玻璃碳;而在高压高温条件下,玻璃碳就直接转变成了金刚石。最新研究则在中等温度下对玻璃碳加高压,这样既能使玻璃碳发生相变形成新型碳材料,温度又不足以使它变成金刚石。压缩玻璃碳有以下特性:它是与石墨类似的轻质材料,单轴压缩强度是通常金属及合金材料的5倍以上,也远大于一般陶瓷材料;比强度(即强度-重量比)极高,是碳纤维、聚晶金刚石、碳化硅和碳化硼陶瓷的2倍以上;硬度极高,可轻松刻划高硬度的碳化硅单晶片;具有很高的弹性恢复性,明显高于普通金属和陶瓷,甚至高于高弹性的形状记忆合金及有机橡胶等;此外还具有导电性。
