Unexpected stoichiometry in Sn-Se system - Dr. Kuo Li
MARCH 30, 2017
Scientists from HPSTAR and The University of Hong Kong combined in situ synchrotron x-ray diffraction and density functional theory and evolutionary algorithms to examine the high-pressure structural behavior of Sn-Se system. The team observed a new compound with an unexpected stoichiometry in the Sn-Se binary system. The research is published in the March 28th edition of Physical Review Letters.
Under ambient conditions, Sn atoms exhibit oxidation states of +2 or +4, and Se atoms often have a -2 oxidation number, so the conventional binary compounds observed in the tin-selenium system are SnSe and SnSe2, representing the typical stoichiometries in the IV-VI group.
While the team of scientists found that high pressure would change the conventional stoichiometry in the Sn-Se system just like what it did in the other binary systems, eg. Na-Cl, Ca-C and etc.
Extremely high pressure will completely alters elements’ chemistry, and we will get more exotic compounds that we couldn’t under ambient conditions. “So we could even say that pressure will make a new chemistry world compared to ambient pressure. This raises questions about chemistry and how elements behave beyond the world we know”, said Dr. Kuo Li, a chemist of HPSTAR.
Sn-Se compounds attracted the group’s interests because they are good semiconductors and widely applied in photovoltaic, thermoelectric and memory-switching devices. They are aiming to find other exotic compounds in the Sn-Se system because exotic compounds not only expand our understanding of chemistry but may find new practical applications in future.
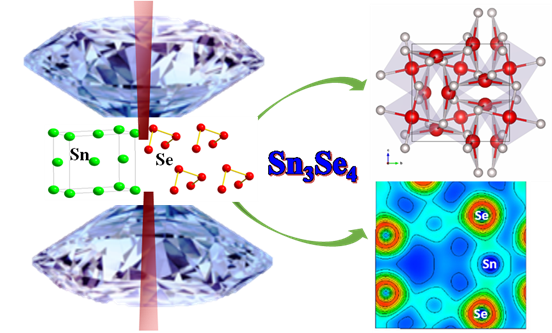
First, the team used theoretical calculation to find stable compounds in the Sn-Se system up to 400 thousands atmosphere pressure and a new compound with an unexpected stoichiometry 3:4 was predicted to be stable at high pressure. “Unlike the layered crystal structures of SnSe and SnSe2, Sn3Se4 has a more densely packed crystal structure with potential high hardness, good thermoelectricity, ferromagnetism or superconductivity.
“Fortunately, the large pressure range where Sn3Se4 is energetically preferable makes it possible to be synthesized. So inspired by theoretical predications, HPSTAR team managed to synthesized the predicted new compound Sn3Se4 at some 160 thousands atmosphere pressures and 900 oC using a laser-heated diamond anvil cell, and collected the corresponding X-ray diffraction data successfully.
“Our result is one more perfect collaboration between experimental and theoretical materials research," said Dr. Yue Chen of The University of Hong Kong, the co-led author. “Many novel materials were predicted everyday by the theoretical calculations but most of them are still need to be realized by experiment.”
From further electronic structure calculation, Sn3Se4 is predicted to be metallic and exhibit a superconducting transition at low temperature and low pressure, which is different from the conventional SnSe and SnSe2 semiconductors. Moreover, Sn3Se4 shows a mixed nature of chemical bonds from electron density and Bader charge analysis. “The successful synthesis of Sn3Se4 paves the way for the discovery of other IV-VI compounds with non-conventional stoichiometries and novel properties,” added Dr. Lijuan Wang of HPSTAR, a co-author of the study who conducted the experimental work.
Caption: Novel pressure-stabilized superconducting tin selenide with unexpected stoichiometry. Courtesy of Lijuan Wang.
压力作为一个新的维度,通过改变原子间距,电荷分布,从而改变物质的结构,形成具有新性质的高压相。近几年来的理论研究和实验表明,在高压条件下,压力还可以改变元素的化学配比,从而形成经典化学中不可能的化合物。比如Na-Cl, Ca-C等体系中新型化合物的发现,惰性气体化合物,Ar(H2)2, Na2He等等的发现突破了人们对原有化学知识的认知。这些在常压条件下不可能存在的化合物,在高压下都能被合成并稳定存在。在Sn-Se二元体系中,人们通常只观测到SnSe和SnSe2两种化合物。北京高压科学研究中心的李阔研究员及香港大学的陈粤教授通过理论预测及高压实验合成了此二元体系的另一新型化学组成的化合物-Sn3Se4。相关结果发表于近期的《物理评论快报》上。
