Observation of sodium polyhydrides under pressure- Drs Duckyoung Kim & Ho-Kwang Mao
JULY 29, 2016
Combining synchrotron x-ray diffraction and Raman spectroscopy, a team co-led by HPSTAR scientist, Duckyoung Kim, report the first observation of formation of sodium polyhydrides (NaH3 and NaH7) between two diamond tips. These results are applicable to the design of new energetic solids and high-temperature superconductors based on hydrogen rich materials. These findings were reported in Nature Communications (doi:10.1038/ncomms12267).
A glance at the periodic table suggests that hydrogen should be a metal at sufficiently high densities because it is in the same column as the alkali metals — an idea that was proposed over 80 years ago by Wigner and Huntington. However, metallic solid hydrogen hasn’t been observed experimentally even it has been compressed to a record high-pressure of ~385 GPa from static techniques.
To circumvent the difficulty generating ultimately high pressure, it was suggested that metallic phases of hydrogen-rich materials (polyhydrides) might provide a proxy of metallic hydrogen at relatively low pressure. The polyhydrides are predicted to require lower pressure to metalize than pure hydrogen due to the chemical precompression caused by heavier elements.
Molecular H2 is the most abundant form of hydrogen. It is also well known that isolated H3+ molecule (three protons and two electrons) forms equilateral triangle shapes. Similarly, while the binding energy is weak, H3- molecule (three protons and four electrons) was predicted to be a linear chain. In polyhydrides under pressure, it is likely that metal elements donate electrons to hydrogens and provide an excellent platform to study ionic hydrogen molecules in solids.
However, such high pressure experiments are very challenging since the hydrogen often diffuse into diamonds under high pressure-temperature conditions. Most of these kinds of experiments will result in failure due to diamond breakage.
The research team-Viktor V. Struzhkin, DuckYoung Kim, Elissaios Stavrou, Takaki Muramatsu, Ho-kwang Mao, Chris J. Pickard, Richard J. Needs, Vitali B. Prakapenka, and Alexander F.Goncharov, challenged the difficulties and recreated the experiments by squeezing Na/NaH and hydrogen between two diamond tips, and heated the sample by lasers to raise temperature.
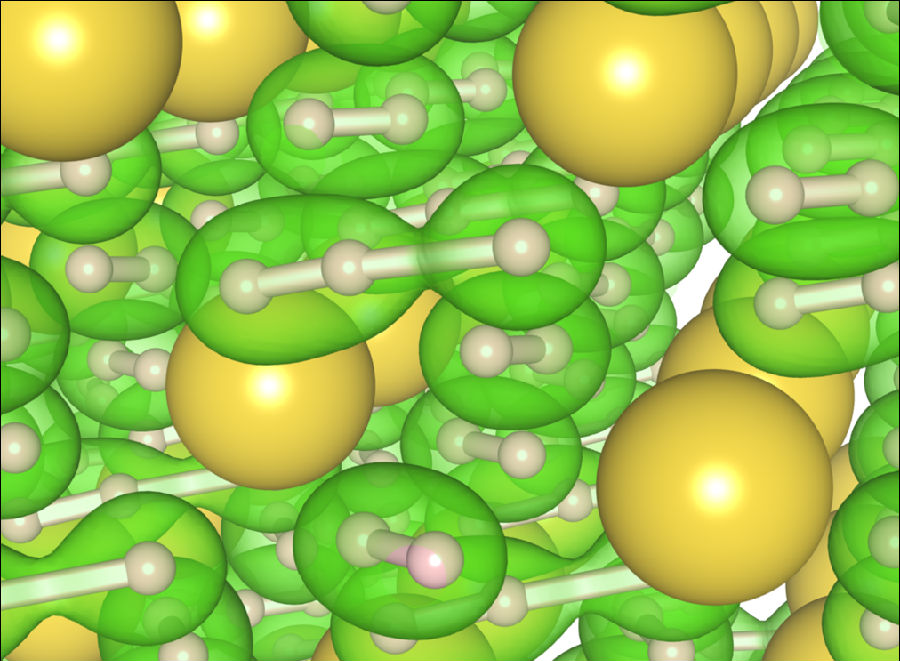 They succeeded to produce sodium polyhydrides above 400,000 times normal atmospheric pressure (40 GPa) and 2000 degrees Kelvin, enabling the characterization of the NaHn phases using Raman spectroscopy and x-ray diffraction.For the first time, they identified the existence of NaH3 and NaH7 under pressure. Interestingly, by a careful comparison of vibrational motions of hydrogen molecules, they found that H3- unit in NaH7 exhibits a much reduced frequency than solid hydrogen. As a dissociation of H2 molecule is directly related to metallization of hydrogen, the frequency softening implies a promising metallization of sodium polyhydrides at experimentally tractable pressure regime.
They succeeded to produce sodium polyhydrides above 400,000 times normal atmospheric pressure (40 GPa) and 2000 degrees Kelvin, enabling the characterization of the NaHn phases using Raman spectroscopy and x-ray diffraction.For the first time, they identified the existence of NaH3 and NaH7 under pressure. Interestingly, by a careful comparison of vibrational motions of hydrogen molecules, they found that H3- unit in NaH7 exhibits a much reduced frequency than solid hydrogen. As a dissociation of H2 molecule is directly related to metallization of hydrogen, the frequency softening implies a promising metallization of sodium polyhydrides at experimentally tractable pressure regime.
“However, the potential metastable phases of the polyhydrides should be carefully explored in the future studies, said Kim. “Multigrain method could be used to distinguish the NaH3 and NaH7 phases and give more precise structure in the future study,” added Mao, a senior author of the study and director of HPSTAR.
“Polyhydrides of alkali metals open a new class of materials with pressure stabilized symmetric, linear, H3- sublattice (three centered four electron bond) for future investigation”, Kim say. “This study strengths our belief to find out the high-temperature superconductor in alkli polyhydrides with lower technical requirement.
Caption: The image shows crystal structure of NaH7 with H2 and H3 units at 40 GPa. The green colored volumes exhibits an iso-surface of a certain-level charge density in hydrogen, curtesy of Duckyoung Kim.
