
New study led by Drs. Xujie Lü and Chuanlong Lin at HPSTAR has, for the first time, successfully synthesized a metallic cubic CsCuBr₃ perovskite under high-pressure and high-temperature conditions, revealing its unusual structural evolution mechanism. The study, titled “Anomalous Metallic Cubic CsCuBr₃ Perovskites: Pressure- and Temperature-Driven Suppression of Jahn–Teller Distortion”, was published in the Journal of the American Chemical Society (JACS).
Halide perovskites have garnered significant attention due to their excellent optoelectronic properties, with applications in solar cells, light-emitting diodes, and photodetectors. However, most halide perovskites reported to date are based on main-group metal cations (such as Pb²⁺, Sn²⁺, and Ge²⁺), which typically exhibit semiconducting behavior due to their electronic structures governed by s and p orbitals. In contrast, metallic behavior in halide perovskites remains rare. To address this limitation, transition-metal cations, particularly Cu2+ (with its 3d⁹ configuration and partially filled d orbitals), have been considered promising candidates for metallic perovskite behavior. However, the inherent strong Jahn–Teller distortion of Cu2+ usually disrupts the three-dimensional (3D) octahedral framework, making it extremely difficult to synthesize stable 3D Cu²⁺-based perovskites
Under ambient conditions, CsCuBr₃ does not adopt a perovskite structure but instead crystallizes into a low-dimensional orthorhombic chain-like structure. Thisbehavior is attributed to the strong Jahn–Teller distortion associated with 3d⁹ electronic configuration of Cu²+, which severely deforms the polyhedral framework. Remarkably, this distorted structure remains stable even under compression up to 40 GPa at room temperature. By simultaneously applying high pressure and temperature, the researchers successfully synthesized cubic perovskite phase (Pm-3m). The cubic CsCuBr₃ features a complete corner-sharing CuBr₆ octahedral network, free from Jahn–Teller distortion. Even more surprisingly, instead of exhibiting semiconducting behavior typical of halide perovskites, this cubic phase exhibits metallic conductivity, with a resistance just a few tens of milliohms at 18 GPa (Figure 1).
First-principles calculations further elucidated the electronic origin of this anoumalous metallic behavior. Under compression, strong hybridization between Cu-3d and Br-4p orbitals occurs near the Fermi level, enhancing orbital degeneracy and electron delocalization—key factors driving the emergence of metallicity. Notably, this cubic phase remains stable down to approximately 4 GPa at room temperature and can even be maintained near ambient pressure at cryogenic temperatures. This stability suggests that it may be possible to reserve this unusual perovskite phase under milder conditions, enabling further exploration of its electronic states and potential applications.
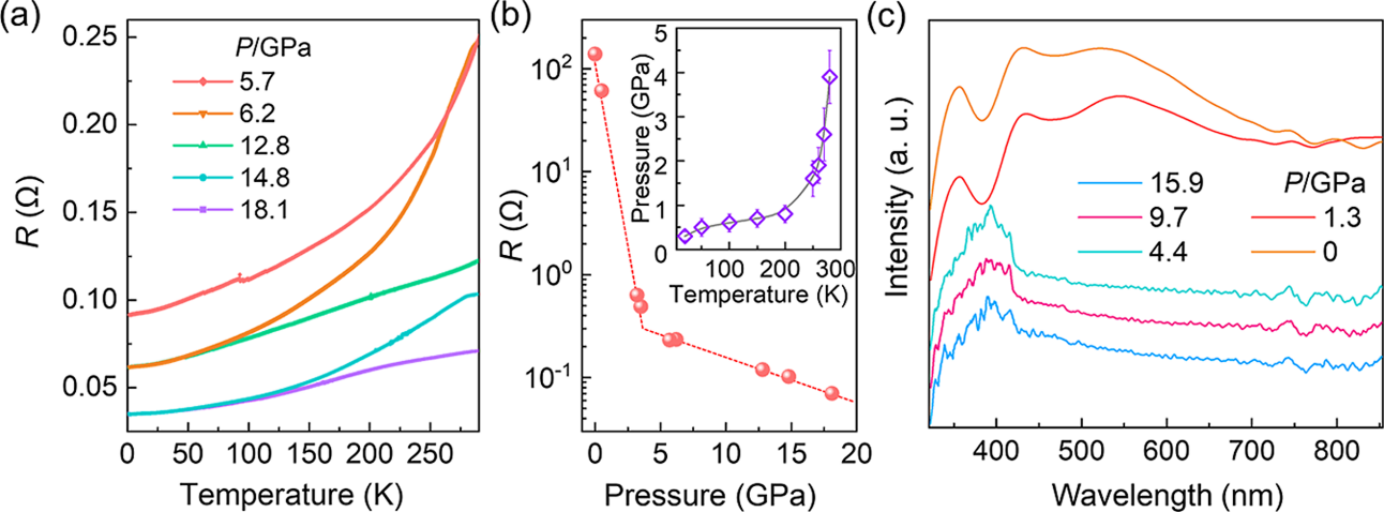 Figure 1. Electrical and optical properties of the cubic structure of CsCuBr3. (a) Resistance of the cubic phase as a function of temperature during decompression. (b) Resistance as a function of pressure at 280 K. Inset: Pressure at which a significant resistance change occurs as a function of temperature. (c) Pressure-dependent UV−vis absorption during decompression.
Figure 1. Electrical and optical properties of the cubic structure of CsCuBr3. (a) Resistance of the cubic phase as a function of temperature during decompression. (b) Resistance as a function of pressure at 280 K. Inset: Pressure at which a significant resistance change occurs as a function of temperature. (c) Pressure-dependent UV−vis absorption during decompression.
北京高压科学研究中心(HPSTAR)吕旭杰研究员和林传龙研究员团队首次在高温高压条件下合成出了金属性的立方 CsCuBr₃ 钙钛矿,并揭示了其独特的结构演变机制。相关研究成果以“Anomalous Metallic Cubic CsCuBr₃ Perovskites: Pressure- and Temperature-Driven Suppression of Jahn–Teller Distortion”为题,发表于《美国化学会志》