A team of scientists led by Dr. Lei Su from HPSTAR has succeeded in synthesizing the novel state of sulfur - the amorphous sulfur chains. This new state was evading the researches for quite some time but was finally reached by utilizing the deceptively simple bit very efficient fast compression technique. The discovery adds yet another level of complexity to already relatively complicated phase diagram of sulfur. The research published Nature Communications introduces a cutting-edge new technology of fast compression at elevated temperatures.
Yellow and stinky sulfur has been in focus of the high-pressure research for the long time, first, studied using the multi anvil press and later using the diamond anvil cell. The interest comes from the pressure induced complexity - sulfur has one of the richest, as far as the different physical phenomena concerned, phase diagram among the elements. Due to the sulfur’s atoms’ propensity to form molecules and/or polymeric chains of various sizes and configuration, elemental sulfur possesses more allotropes and polymorphs than any other element at ambient conditions. This variability of the starting building blocks is partially responsible for its rich and fascinating phase diagram, with pressure and temperature changing the states of sulfur from insulating molecular rings and chains to semiconducting low- and high-density amorphous configurations to incommensurate superconducting metallic atomic phase.
The paper demonstrated that adding another thermodynamical parameter, namely time, can fundamentally alter the properties of this fascinating element and create yet anotherstate. The study has shown that the rapid compression of liquid sulfur in a ring state can effectively break the molecular ring structure, forming a glassy polymeric state of pure-chain molecules (Am-SP). This solid disordered chain state appears to be (meta)stable in the P-T region usually associated with phase I made up of S8. The elemental sulfur glass, made up from one of the simplest building blocks, offers a unique prospect to study the structure and property relationships of various other phases of sulfur and their interactions.
This feast was achieved by combining the old horse of the high-pressure research diamond anvil cell with the piezo-electrical motor, which can increase pressure on the level of several tens of TPa per second (10 M Atm/s) at elevated temperatures. The technique was developed by Drs. Shi and Su and the paper represents one of the first cases where the technique was successfully used beyond simple proof of concept uncovering the new science. “The classical way to create the glassy state is temperature quenching. But due to the huge, relative to the sample, mass of the diamond anvil cell one cannot quench fast enough so sulfur does not revert back to cycling rings or crystalline chain phases where you start,” said the first author Dr. Shi. “Fast compression on the other side provides us with the tool, which can easily break the rings and is so fast that the resulting chains do not have time to coalesce back into the rings due to being trapped at high density solid state after the pressure jump,” added Dr. Shi.
"Thermal quenching has long been the primary method for ‘freezing’ the disordered structure of a liquid to produce amorphous (glassy) states that reflect the molecular configuration of the parent liquid,” explained Dr. Su. “In contrast, our rapid compression technique not only vitrifies liquid sulfur but also opens the S8 molecular rings, forcing a ring-to-chain transformation and producing a glassy sulfur composed of polymeric chains. This highlights a significant distinction from traditional quenching methods.”
Changes in molecular structure often trigger phase transitions, but this study reveals phase transitions can drive molecular structure transformations. This ability to synthesize amorphous materials with modified molecular configurations or short- to medium-range order presents a critical advantage over conventional thermal quenching methods, offering a promising pathway for the synthesis of novel functional materials.
The fast compression technique performed at any temperature effectively acts like thermal quenching, opening up possibilities in high pressure synthesis by providing an easy and fast way of changing the fundamental thermodynamical parameter. By combining the fast compression with time-resolved spectroscopies, both optical and x-ray, one can study the phase formation, molecular dissociation/recombination and particularly melting/solidification at high pressure. The rapid compression method can introduce considerable density changes and drive subtle structure transformations in the melt, resulting in amorphous products with distinct properties. This ability to produce amorphous materials with modified molecular structures or short- to medium-range order underscores a key difference from the temperature quenching process, providing an effective way to synthesize potentially useful materials.
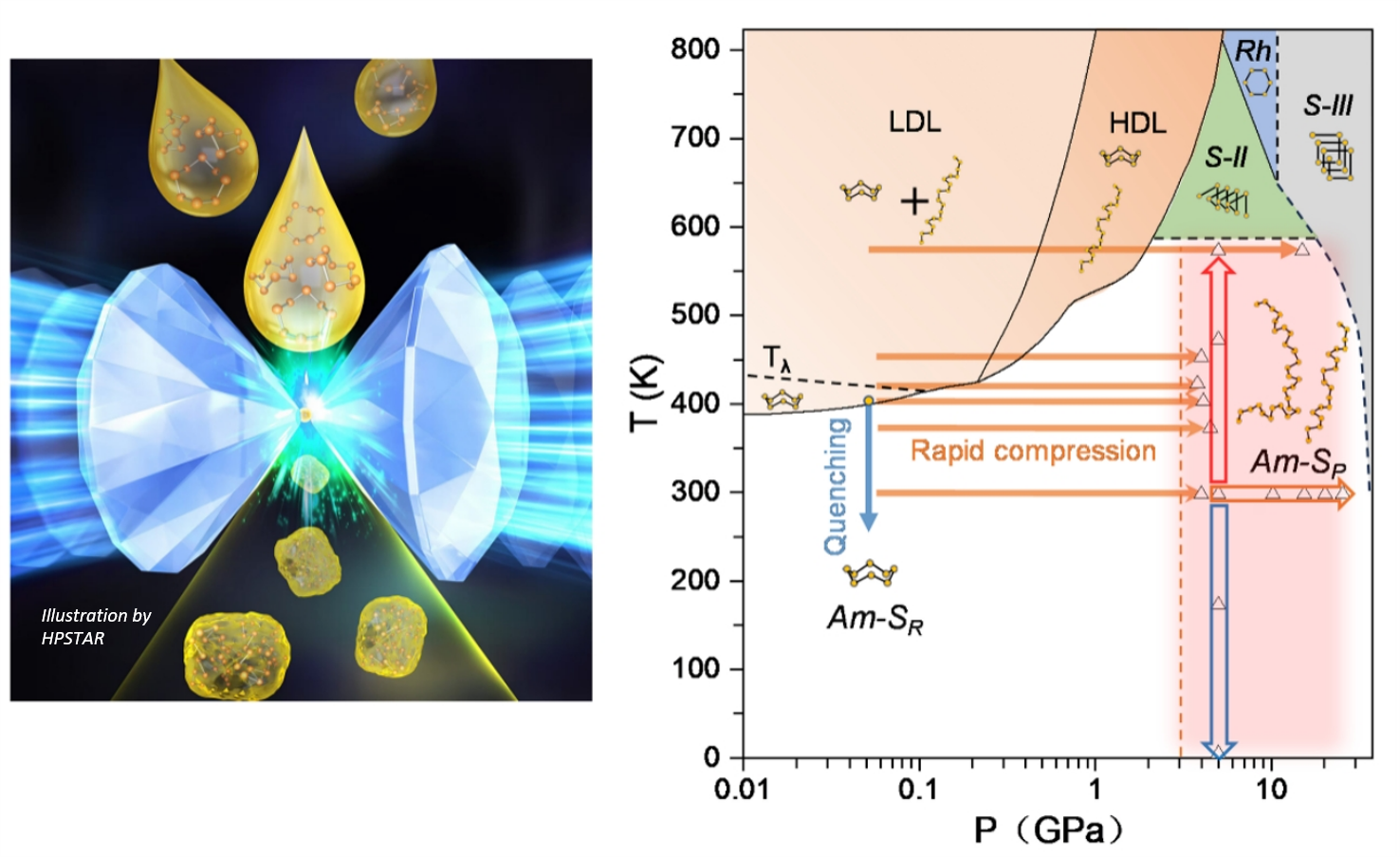 Caption: The rapid compression of liquid sulfur to effectively break the ring structures and form an elemental sulfur glass of pure chain molecules (Am-SP).
Caption: The rapid compression of liquid sulfur to effectively break the ring structures and form an elemental sulfur glass of pure chain molecules (Am-SP).
近日,由北京高压科学研究中心、中国科学院化学研究所、燕山大学、南开大学、上海前瞻物质科学研究院等组成的研究小组利用快速压缩技术,合成了一种具有全链结构的非晶硫(Am-SP)。相关成果以“Sulfur chains glass formed by fast compression”为题发表于近期的《 自然|通讯》。文章链接:https://www.nature.com/articles/s41467-024-55028-w。
