98% of the Universe as Unreactive as Ever - Dr. Ross Howie
NOVEMBER 13, 2018
Extreme conditions are very common in the universe, from the cold vacuum of deep space to the elevated temperatures and pressures within planets. The gas-giant planets, Jupiter and Saturn, consist primarily of hydrogen and helium, whilst the nitrogen-hydrogen bonded compound ammonia, is abundant on Saturns icy moons, Titan and Enceladus.
In a study published in Physical Review Letters, the authors unequivocally demonstrate that hydrogen and helium are remarkably unreactive and immiscible with each other, even at pressures in excess of 2.5 million times atmospheric pressure. This behaviour is in stark contrast to mixtures of molecular nitrogen and hydrogen, which readily form a variety of compounds. Interestingly, when mixed at high densities, the nitrogen-hydrogen mixtures do not react to form ammonia. Instead, the mixture forms a solid comprised of nitrogen and hydrogen molecules held together by weak van der Waals interactions. Only at half a million times atmospheric pressure does the density become high enough for the molecular bonds to break and form an N-H bonded compound. This combined study gives important insight into the reactivity of hydrogen with simple elements and molecules at extreme densities.
“There has always been significant interest in understanding the interactions between hydrogen with helium given their importance for planetary studies. This area of research was given recent impetus with the unprecedented claim that hydrogen and helium react at high pressure. By combining data accumulated over an 8 year period, our collaborative effort was able to rule out any chemical reaction and attribute this finding to nitrogen contamination of the groups gas mixture.” said Dr Ross Howie, who led this study. “Our study provides a valuable lesson; to never rule out the most simple explanations of results.”
The research team comprised of HPSTAR members Mary-Ellen Donnelly, Mengnan Wang, Philip Dalladay-Simpson, Cheng Ji, Eugene Gregoryanz, Ho-Kwang Mao and Ross Howie, together with researchers from the University of Edinburgh.
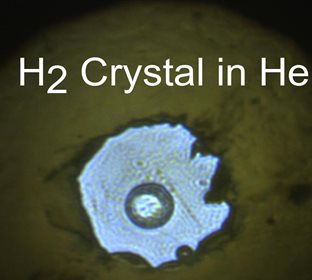
Caption: The single crystal of H2, located in the center of the sample chamber, is clearly phase separated from the surrounding He medium.
氦作为一种惰性气体,被证实其化学惰性在极端条件下会丧失,会与金属钠发生化学反应。那么作为宇宙中含量最丰富的两种元素—氢,氦,是否也会有化合物存在呢?北京高压科学研究中心的Ross Howie研究员带领的研究小组一直致力于氢及其化合物在极端条件下的物性研究。在他带领的新的研究中将氢,氦以不同比例混合后加压至两百多万大气压,发现氢依然保持原有的分子状态,而氦气的化学惰性也没有因为氢分子的引入而被破坏。该研究结果证实了近期J.Lim和C.-S.Yoo所报导的氢-氦化合物合成可能是由于初始气体混合的过程中或装样时空气中氮气的混入导致了原始气体样品的污染。
