Hydrogen heavyweight found for methane-hydrogen compounds - Dr. Ross Howie
MAY 28, 2022
Hydrogen and methane are, besides water, the most prevalent small molecules in the outer solar system. At high pressure, they tend to form inclusion compounds and their signatures have emerged in various studies investigating hydrocarbons at deep-Earth conditions and synthesizing high-temperature superconducting hydrides, but were never formally identified.
In recent work published as an Editors’ Suggestion in Physical Review Letters, a team led by Dr. Ross Howie, and including HPSTAR (past and present) members Dr. Umbertoluca Ranieri, Dr. Mary-Ellen Donnelly, Dr. Philip Dalladay-Simpson, Prof. Eugene Gregoryanz, and Miss Huixin Hu report the high-pressure formation of the most H2-rich compounds found to date, (CH4)3(H2)25, CH4(H2)2 and (CH4)2H2. Forming above 5 GPa, the compounds exhibit remarkable stability exceeding 160 GPa—about 1.6 million times the atmospheric pressure on Earth.
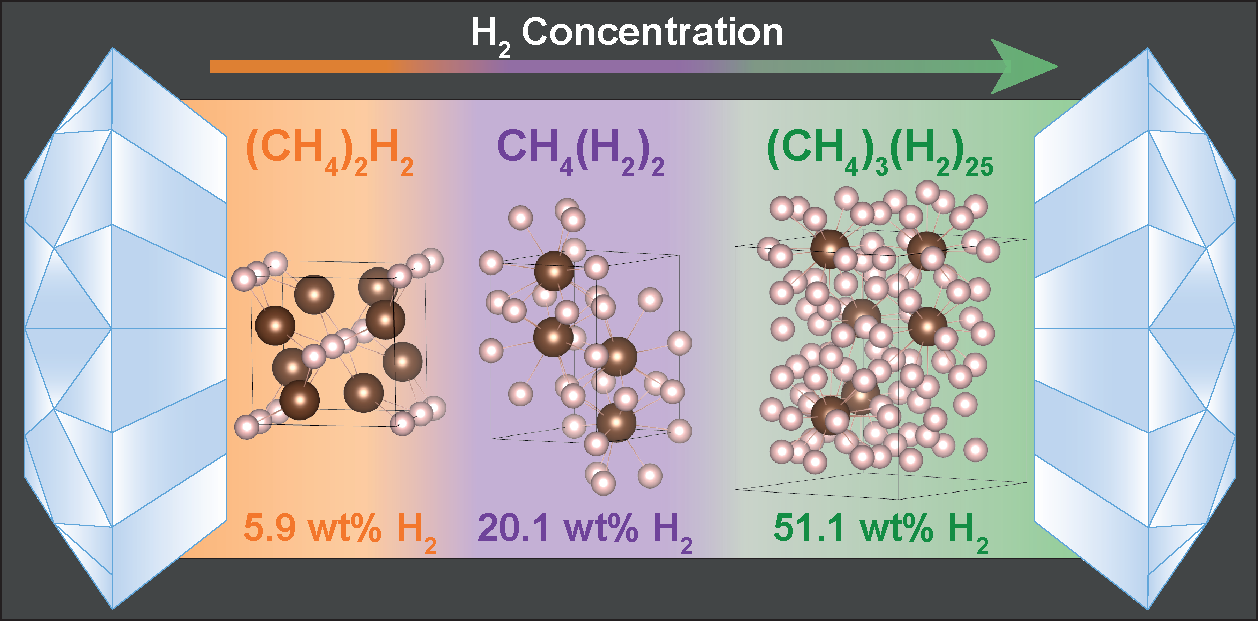
With a completely unique composition, (CH4)3(H2)25 contains the most molecular hydrogen (over 50 wt%) of any known compound. The team found that the melting temperatures of each compound are substantially different from either methane or hydrogen. From a planetary context, this could influence critical properties of planetary matter, such as thermal conductivities and viscosities. From a materials science context, methane-rich mixtures yields (CH4)2H2, which exhibits an extremely high intramolecular vibrational frequency combined with a high Debye temperature, both of which are promising ingredients for high-Tc superconductivity.
“Methane-hydrogen compounds have been known to form for the past 25 years. However recent technological advances have allowed us to explore this hydrogen-rich system comprehensively,” said Dr Ross Howie, “The challenge ahead will be stabilizing these H2-rich compounds at lower, more ‘useful’ pressures”.
Media report:
Physics Synopsis: https://physics.aps.org/articles/v15/s69.
氢气和甲烷是外太阳系除水以外普遍存在的小分子,它们在极端条件下的相互作用是理解地球等行星的演化和内部动力学的关键。氢在极端温度,压力条件下会与许多物质发生化学反应而形成化合物,比如铁,硫。理论计算表明,氢与甲烷也会在高压下发生反应形成范德瓦尔斯化合物,然而对于此反应一直缺乏实验方面得系统研究。近日,北京高压科学研究中心得Ross Howie 研究员带领的研究小组使用高压同步x射线衍射和拉曼光谱实验并结合理论计算,探索甲烷和氢气在百万大气压下的化学反应。他们发现甲烷与氢气在高压下会形成了三种非常稳定的富氢化合物: (CH4)3(H2)25, CH4(H2)2 和(CH4)2H2,其中(CH4)3(H2)25的氢含量高达51.1 wt %,是所有已知物质中氢含量最高的,有望用于储氢研究;(CH4)2H2表现出极高的分子内振动频率和较高的德拜温度,具备了高温超导的两大主要潜在特征;并且,每种化合物的熔化温度都与甲烷或氢气有本质上的不同,这可能会影响行星的关键性质,如热导率和粘度。相关研究发表于近期的Physical Review Letters, 并被选为”编辑推荐“文章。
