Universal Transitions in Hydrogen Bonds under Extreme Conditions - Dr. Thomas Meier
JUNE 1, 2022
Hydrogen bonds are ubiquitous in nature, they form the backbone of living matter by stabilizing DNA molecules, they are the key for the existence and discovery of novel and exciting materials and their study is a driving force in contemporary condensed matter research. Systems stabilized by H-bonds often exhibit an extraordinary plethora of physical phenomena, like structural diversity, superionicity or high-temperature superconductivity. These phenomena often occur close to the so-called symmetrisation of the hydrogen bonds, i.e. when the hydrogen atoms are located in the geometric center between the two heavier bonding atoms, see for example figure 1.
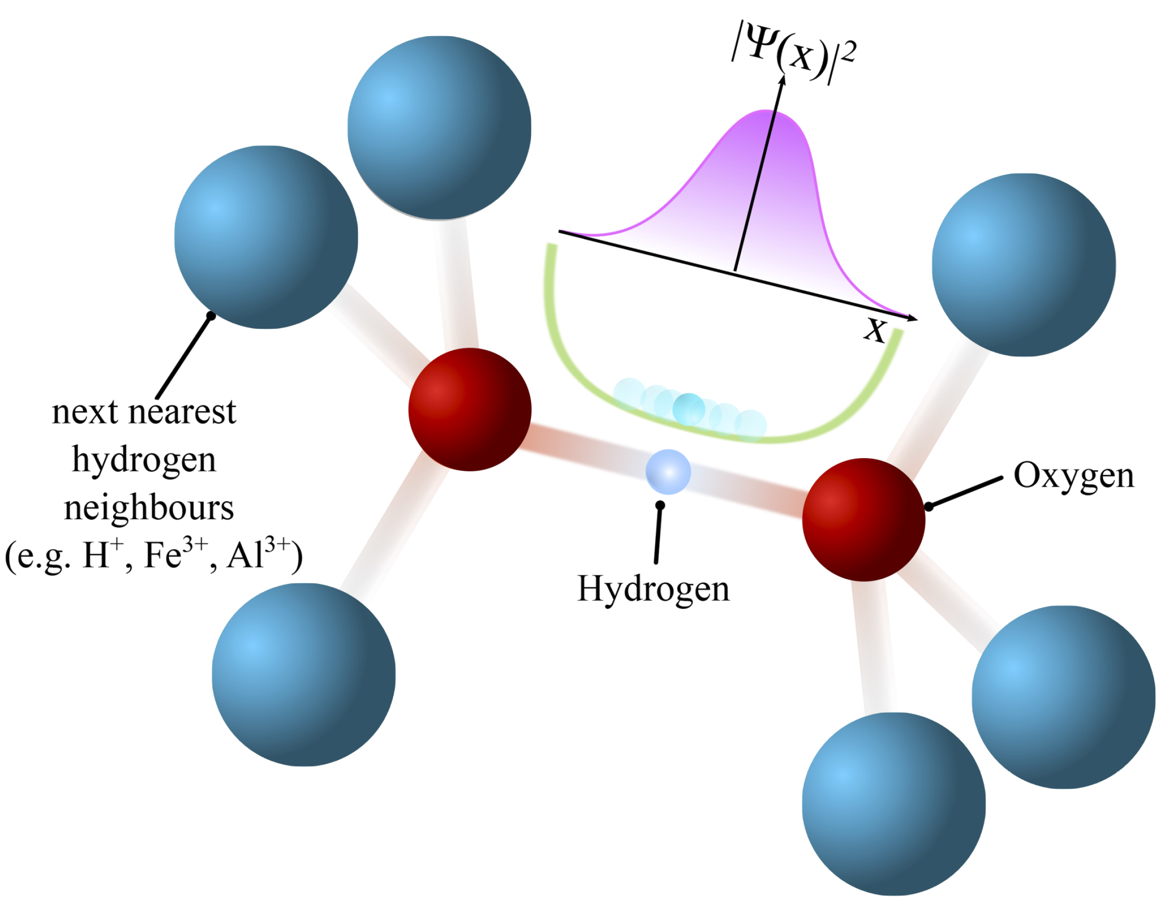
Figure 1: Hydrogen bonds are often formed between two homo- or heteroatomic neighbors (here oxygen atoms) which are shared by other heavier atoms. The resulting energy potential in the space between the oxygen atoms (green line) represents the point of lowest energy for hydrogen atoms to coordinate in the hydrogen bond.
However, researchers were lacking a deeper understanding of the underlying physical principles of hydrogen bond symmetrisations in those materials, caused by the inherent challenges posed by directly investigating hydrogen atoms in Diamond Anvil Cells – the standard method to achieve extreme conditions similar to the center of the Earth – using diffraction methods or other spectroscopic techniques.
An international research group led by Dr. Thomas Meier from the Center of High Pressure Science and Technology Advanced Research (HPSTAR) in Beijing, where able to discover a unifying relationship in a number of distinct samples under extreme pressures. In particular, they were able to observe Hydrogen bond symmetrisations in the high-pressure polymorphs ice VII and X, magnesium silicate phase D, as well as in iron bearing and iron free aluminium oxyhydroxides. Employing a novel method of performing Nuclear Magnetic Resonance spectroscopy - a widespread diagnostic for Chemistry and Materials Science – under high pressures, whose development is spear-headed by Dr. Meier and his colleagues, the researchers found that H-bond symmetrisations occur at almost identical distances of the main constituents of the bonds, the oxygen atoms. This transition was found to be fully independent of the chemical, structural or even quantum-mechanical nature of the sample or the hydrogen bond environments investigated.
"This effect was fully unexpected for us, in particular because all samples we looked at are considered very different from one another, both chemically and structurally.” said Dr. Meier and continues “This observation could unify and explain numerous different and often contradictory results from other high-pressure groups working in this field, which for example, correlate hydrogen bond symmetrisations either to structural phase transitions, rearrangements of electron spin statistics or both. But it turns out that none of these interpretations seems to be correct and H-bond symmetrisations are really a stand-alone physical phenomenon.”
Dr. Florian Trybel from Linköping University remarked: "With this discovery, we are one step closer to understand the physical consequences of this elusive but wide-spread phenomenon.”
The researchers involved in this study form a multi-national group around Dr. Thomas Meier and Dr. Takayuki Ishii (both from HPSTAR) as well as scientists from Sweden, the United Kingdom and Germany.
The researchers describe their work in an article published in the Journal Nature Communications.
常压条件下,含有线性氢键的物质中的氢键是非对称的,随着压力的增加,非对称的氢键往往会趋于对称。高压下不同体系中氢键对称化的过程中通常会伴随着不同的压缩性能,或者输运性质等物性的改变,因此在传统的观点中,研究者往往将氢键对称化与物质的结构相变、高低自旋转变等变化关联起来,然而这些关联性解释往往又自相矛盾。为了深入探索关于氢键对称的相关问题,近日,北京高压科学研究中心(HPSTAR)的Thomas Meier研究员带领的国际研究小组,对一系列不同样品进行了高压原位核磁共振光谱测量,发现了关于氢键对称化的统一规律:不同体系中氢键的对称都发生在氢原子流动最快的临界点,并且氢键对称是一个完全独立的物理现象,与结构或自旋相变并不相关。相关研究发表于近期的Nature Communications上。
