Discovery of carbon-based strongest and hardest amorphous material – Dr. Alex Soldatov
AUGUST 20, 2021
Carbon exhibits a vast variety of allotropic forms stemming from its different chemical bonding motifs - from well-known graphite and diamond, to fullerenes and carbon nanotubes build of nanometer-sized structural units. In this row diamond possesses a “special” place being the hardest known natural material on Earth. This status-quo remained until a research team from Yanshan university led by Prof. Yongjun Tian in collaboration with Prof. Alex Soldatov of HPSTAR produced for the first time a nearly pure sp3-bonded amorphous carbon that is harder than- and equally strong as natural diamond. The material termed AM-III, is a transparent semiconductor with a bandgap of 2.2 eV. It surpasses in hardness and fracture toughness all known amorphous materials. This work is presented in the August issue of National Science Review.
Needless to say challenging the superior hardness of diamond ever remains a quest for materials scientists. Even though the occasional reports claimed synthesis of a material comparable in hardness to natural diamond, capable of scratching it, they were inconclusive and only special structural forms of diamond itself produced in the lab - nano-polycrystalline and highly enriched in twin defects - demonstrate enhanced hardness compared to natural diamond crystals.
In this effort, a series of amorphous carbon phases were recovered from compressing fullerene C60 at high temperature and 25 GPa, the pressure never explored before for buckyballs transformation to other carbon forms at high p,T. It turned out to be of a paramount importance as it is at this pressure level that the sp2-bonded carbon atoms from residual graphene clusters formed upon C60 collapse are “squeezed out” in the structure – transform into sp3-bonded carbons tetragonally arranged in clusters. Among these phases the AM-III, with approaching diamond density of 3.30 g/cm3, exhibits a remarkable combination of physical properties: the Vickers hardness (HV) is up to 113 GPa and the nanoindentation hardness is 103 GPa, which surpasses the record of 80.2 GPa held until now by the diamond-like amorphous carbon (DLC) films; the compressive strength of its micro-pillars reaches 70 GPa, which is comparable to that of diamond. Thus, AM-III is the hardest and strongest amorphous material found so far, the long-time sought ultrahard DLC material synthesized for the first time in a bulk form. In addition, it is an optically transparent semiconductor with a bandgap 2.2 eV, which is close to that of the most common amorphous silicon films.
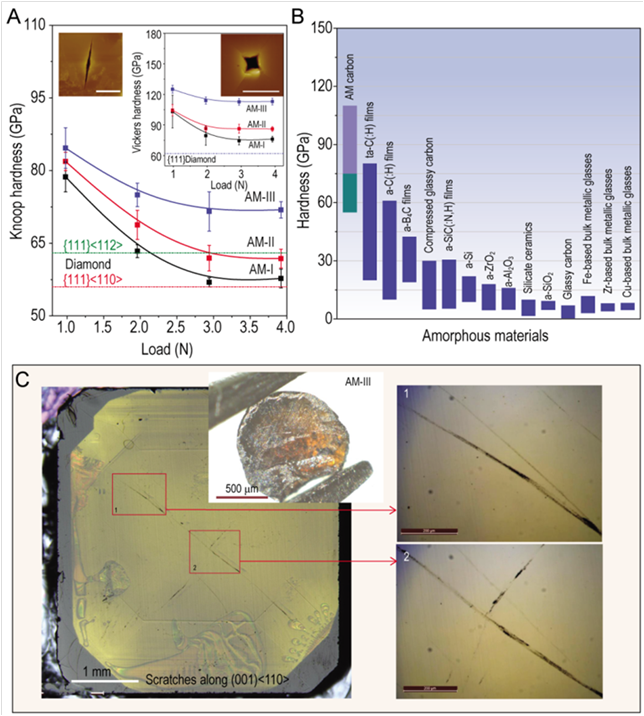
Caption: (A) hardness of synthesized carbon (AM)- and (B) other amorphous materials; (C) scratches made by AM-III on (001) face of natural diamond.
“We believe such combination of the outstanding properties, Dr. Soldatov says, is due to a very particular short range order featured by this nearly pure sp3-carbon AM-III phase that is comprised of the disordered clusters of sp3-bonded carbons with tetragonal diamond-like structure (matrix) with embedded fused aromatic rings, short chains of residual sp2-carbons interlinking the clusters”.
This research opens up prospective for use of the bulk ultrahard amorhous carbon in, for example, photovoltaics applications that require ultrahigh strength and wear resistance.
