Putting the squeeze on hydrogen-bonding in HCl – Dr. Philip Dalladay-Simpson
SEPTEMBER 6, 2021
In a truly international effort, a team of scientists based in Japan, Australia, UK, USA and China have uncovered the high-pressure behavior of hydrogen chloride (HCl). The project, which was led by Dr. Philip Dalladay-Simpson of HPSTAR, details the significant role that hydrogen still plays even after the pressure-induced symmetrisation of the hydrogen bond in HCl. The team’s findings, which were published in Science Advances, will have implications for our understanding of warm hydrogen-dominant matter under extreme pressures and is therefore highly relevant to planetary interiors.
Under ambient conditions, hydrogen bonding is a fundamental intermolecular interaction responsible for the behavior of numerous molecular crystals, water ice serving as a paradigm. The geometric nature of the interaction creates networks that can rearrange, breakdown, and symmetrize in response to the proximity of neighboring molecules and therefore can be directly probed through compression. However, the fate of these networks at extreme densities – beyond symmetrization – remains difficult to generalize due to limited experimental studies carried out to date. The mantles of the ice giants exist predominately in this ‘post-symmetrized’ regime, making it the norm for hydrogen bonding within our solar system. Due its linear geometry and stability under compression, hydrogen chloride is a simple model and an excellent candidate to gain insights into a post-symmetrised regime.
Through a combination of experiments and first-principles calculations the group explored this archetypal molecule, HCl, to pressures in excess of 256 GPa (more than 4 times the limit of previous experiments). In doing so, the group identified a new post-symmetrised phase of hydrogen-chloride, phase V, characterized by hydrogen atoms becoming mobile and hopping to new lattice sites. Ultimately, under even further compression, alongside with the metallization of HCl, calculations and vibrational characteristics suggest that the hydrogen atoms again become immobilized and frozen into a disordered configuration.
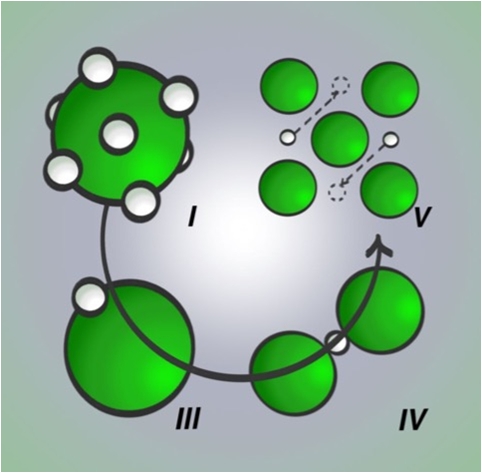
"The fascinating narrative of HCl is how pressure can probe the interplay between hydrogen-bonding and quantum nuclear effects. The former driving the hydrogen atoms into a highly ordered configuration, only for the latter to become the dominant factor at higher compressions reintroducing disorder,” says Dr. Dalladay-Simpson.
Caption: Artistic representation of the phase evolution of HCl under compression at room temperature. Phase I, characterised by hydrogen atom (proton) - disorder as it occupies one of 12 equivalent sites around the chlorine atoms. Phase III, a proton ordered structure as the hydrogen atoms reside at particular lattice sites. Phase IV, hydrogen bond symmetrisation as protons sit at the midpoint between adjacent chlorine atoms. Phase V, re-emergent proton disorder as hydrogen atoms hop to new lattice sites and therefore a room temperature superionic conductor.
