Rethinking the Design of Ionic Conductors Using Meyer–Neldel–Conductivity Plot - Drs. Nana Li and Wenge Yang
MARCH 25, 2021
Recently, a team of scientists co-led by Prof. Shouhang Bo from Shanghai Jiaotong University and Wenge Yang from HPSTAR (Center for High Pressure Science & Technology Advanced Research) proposed to use the Meyer-Neldel-Conductivity plot to design fast ionic conductors with improved conductivity. They stated that the Meyer-Neldel-Conductivity plot is a universal principle that can be used to all ionic conductors for improving ionic conductivity. The results are published in the recent issue of Advanced Energy Materials (DOI: 10.1002/aenm.202100325).
Combustible liquid electrolytes are used for ion transportation in traditional lithium-ion batteries, which has great safety risks. So, in recent years, scientists have aimed to develop solid-state batteries, in which the liquid electrolyte is replaced with non-combustible solid-state materials. The solid-state battery is expected to be widely used as a new generation of batteries in energy storage.
Solid-state electrolytes, as the key part of solid-state batteries, are generally composed of fast ionic conductors with high conductivity. However, compared to liquid electrolytes, fast ionic conductors have much lower conductivity due to their intrinsic solid-state structures. Therefore, to realize the practical application of solid-state batteries, we must develop fast ion conductors with designed conductivity first. So how do we improve the ionic conductivity of fast ionic conductors?
The Arrhenius equation is commonly used in regulating ionic conductivity, and according to this equation, higher ion conductivity corresponds to lower active energy. Researchers have tried to reduce the active energy by various structural modifications to improve ionic conductivity. However, in the Arrhenius equation, the 'prefactor' of the same type of material is assumed to be a constant, but in practice, the 'prefactor' is not a fixed value and is related to multiple parameters in the ion conduction process, following the so-called Meyer-Neldel rule. So, high ionic conductivity cannot be achieved by reducing the active energy alone.
The research team, led by Prof. Bo from Shanghai Jiaotong University in collaboration with Dr. Wenge Yang from HPSTAR, proposed using the Meyer-Neldel-Conductivity plot with a key parameter, Meyer-Neldel energy, to guide the design of new solid-state ionic conductors. They found from the Meyer-Neldel-Conductivity plot that the relative magnitude between the Meyer-Neldel energy and thermal energy at a specific temperature decides the change of ionic conductivity when the activation energy is altered. Furthermore, this principle can be widely used in all ionic conductors, such as Li-, Na-, and Mg-ion conductors.
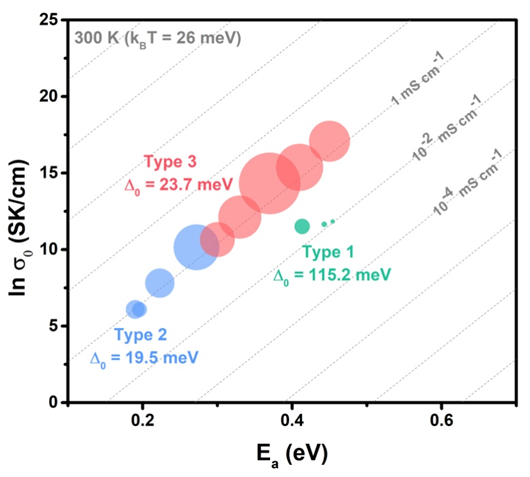 From the Meyer-Neldel-Conductivity Plot, there are three cases: at a given temperature, when Meyer-Neldel energy is greater than thermal energy, lower active energy results in higher conductivity; when Meyer-Neldel energy is less than the thermal energy, lower active energy corresponds to lower conductivity; and when Meyer-Neldel energy is close to thermal energy, the conductivity will not change upon the variation of active energy. Thus, ionic conductors can be categorized into three types, for which different design principles should be adopted depending on the relative magnitude between the Meyer-Neldel energy and thermal energy. Moreover, pressure is a unique and clean method to measure the Meyer-Neldel energy compared to the traditional chemistry method.
From the Meyer-Neldel-Conductivity Plot, there are three cases: at a given temperature, when Meyer-Neldel energy is greater than thermal energy, lower active energy results in higher conductivity; when Meyer-Neldel energy is less than the thermal energy, lower active energy corresponds to lower conductivity; and when Meyer-Neldel energy is close to thermal energy, the conductivity will not change upon the variation of active energy. Thus, ionic conductors can be categorized into three types, for which different design principles should be adopted depending on the relative magnitude between the Meyer-Neldel energy and thermal energy. Moreover, pressure is a unique and clean method to measure the Meyer-Neldel energy compared to the traditional chemistry method.
This work highlights the importance of considering both the Meyer-Neldel energy and activation energy for tuning the ionic conductivity of fast ionic conductors and provides a general conductivity-oriented guideline to develop desired fast ionic conductors.
Caption: Meyer–Neldel–Conductivity Plot for three types of ionic condctors.
传统锂离子电池使用液体电解质而具有很大的安全隐患,因此,近年来,全固态电池引起了人们的广泛关注。所谓全固态电池是指传统电池中的可燃性的液态电解质由非可燃性的固态材料所取代,有望作为新一代电池广泛应用于储能领域。固态电解质作为固态电池的核心,一般是由具有高电导率的固态离子导体材料构成,然而固态离子导体由于其固态晶格的限制,电导率远远低于液态电解质。因此开发具有高离子电导率的固态离子导体是实现全固态电池应用的先决条件。近日,上海交通大学的薄首行教授与北京高压科学研究中心的杨文革研究员带领的研究小组发现不同离子导体的Meyer–Nelde能,热能与离子电导率之间的相关性,并对离子导体提出了一种新的普适的设计思路—Meyer-Neldel-Conductivity图。相关结果发表于Advanced Energy Materials(DOI: 10.1002/aenm.202100325)上。
