Oxygen-excess oxides in Earth's mid-mantle facilitate the ascent of deep oxygen – Drs. Jin Liu, Qingyang Hu and Ho-Kwang Mao
MAY 27, 2020
Subduction of hydrous materials imposes a great influence on the structure, dynamics, and evolution of our planet. However, it is largely unclear how subducting slabs chemically interact with the middle mantle. Recently, an oxygen-excess phase (Mg,Fe)2O3+δ was discovered under the Earth’s middle mantle conditions (~ 1000-2000 km) by a team of scientists from the Center of High Pressure Science and Technology Advanced Research (HPSTAR) and Stanford University. This oxygen-excess phase is fully recoverable to ambient conditions for ex-situ investigation using transmission electron microscopy. It contains ferric iron as in hematite (Fe2O3) which is the most oxidized form of iron on the Earth’s surface, but this new phase holds more oxygen than hematite through interactions between oxygen atoms. The peculiar nature of oxygen in this new phase may revise our view on the mantle redox chemistry. The study is published on National Science Review.
“We employed laboratory techniques to simulate the conditions deep inside the Earth and found an oxygen-excess phase emerged when hydrous mineral assemblages (e.g., ferropericlase mixed with brucite) were exposed to laser heating at pressures greater than 40 million times the atmospheric pressure on the Earth’s surface” said by Dr. Jin Liu from HPTAR. “The formation of this new phase provides strong evidence that water acts as a strong oxidant at high pressure.”
“This phase could coexist with the pyrite-type phase hydrogen-bearing FeO2 at deep mantle conditions, whereas the two phases are distinct in crystal chemistry” added by Dr. Qingyang Hu from HPSTAR. “Unlike the formation of the pyrite-type phase which usually forms at regions found in deep lower mantle and requires a large quantity of water, this oxygen-excess phase can be formed with a moderate amount of water at mid-mantle conditions. The flexible formation conditions make it potentially a more widespread phase at depths greater than 1000 km in Earth’s mantle, occupying almost about the 2/3 portion of the mantle.” Furthermore, this oxygen excess phase can co-exist with the major mantle minerals, bridgmanite and ferropericlase under Earth’s lower-mantle conditions.
“The wide-spread presence of the oxygen-excess phase makes it and other oxygen-enriched oxides an important subject for the full range of future geochemistry and mineral physics studies” suggested by Dr. Ho-kwang Mao, director of HPSTAR. “Remarkably, this new phase is quenchable. As a matter of fact, most compounds synthesized under the lower mantle conditions and quenchable back to ambient conditions, have been discovered and named as minerals such as bridgmanite (Mg,Fe)SiO3 and seifertite SiO2. Hence, this presents an opportunity to search for this oxygen excess phase in nature as diamond inclusions or meteorite shock products.”
The crystal structure of this oxygen excess phase may represent a structure prototype which will accommodate other Earth-abundant components (e.g. Al, Ca, Ti, and Ni). At the same time, the channel space in this oxygen-excess phase could offer a great flexibility not only for excess oxygen, but also for other volatiles (e.g. N, S, F, and Cl). Considering its structural versatility, the new phase could be an important volatile carrier in the deep mantle over geologic time. More importantly, together with excess Fe3+ from the primordial lower mantle, those oxygen-excess materials may have long-termly oxidized the shallow mantle and the crust, which is fundamental to the evolution and habitability of complex life on the Earth’s surface.
These results suggest that the oxygen-excess phase may facilitate oxygen-excess reservoirs out of hydrated slab remnants at depths greater than 1000 km. Oceanic crusts in the mid-mantle thus might deeply regulate the rise of oxygen in Earth's atmosphere and global habitability, like shallowly-recycled fluids. Such intriguing chemistry of deep oxygen sheds light on chemical and dynamic models of mantle slab remnants as well as the interaction and coevolution of Earth's interior and surface.
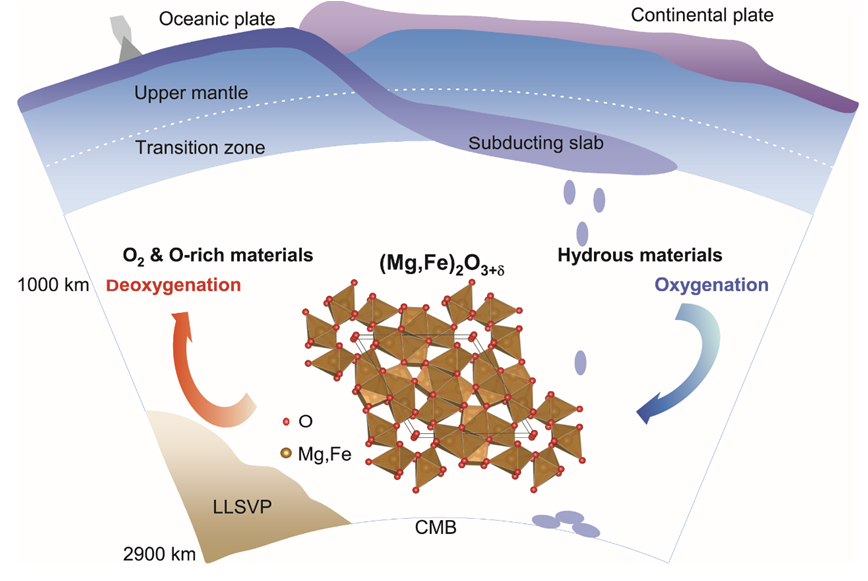
Caption: Deep oxygen factory inside the Earth. A schematic diagram of the deep oxygen factory shows the oxygenation and deoxygenation processes of hydrous mantle materials in the lower mantle across the ~1000 km depth beneath Earth's surface. Under the conditions of Earth’s middle mantle, scientists discovered an oxygen-excess phase, (Mg,Fe)2O3+δ (0<δ<1) that can be formed with under-saturated water at >1000 kilometers depths. Those oxygen-excess materials may have long-termly oxidized the shallow mantle and the crust, which is essential to allow free oxygen to build up in Earth’s atmosphere.
Reference:
Jin Liu, Chenxu Wang, Chaojia Lv, Xiaowan Su, Yijin Liu, Ruilian Tang, Jiuhua Chen, Qingyang Hu, Ho-Kwang Mao, Wendy L Mao, Evidence for oxygenation of Fe-Mg oxides at mid-mantle conditions and the rise of deep oxygen. National Science Review, 2020, nwaa096.
大气中生物赖以生存的氧是如何产生的一直是科学界未解的难题。近年来,北京高压科学研究中心的毛河光院士团队在此方面取得了一系列科学突破,发现在地表1800-2000公里深度以下的深下地幔温度压力条件下含氢过氧化铁FeO2Hx可以稳定存在,揭示了大气中氧气潜在的地球内部来源。然而,在地下1000-2000公里深度(中地幔)是否还存在其他富氧的地幔氧化物呢?近日,该团队的刘锦研究员与胡清扬研究员共同带领的研究团队通过高温高压实验发现了一种新型富氧氧化物,其氧含量高于赤铁矿(Fe2O3)。他们推测该富氧氧化物的晶体结构可以容纳地球内部的其他重要元素,形成稳定的化合物。这一新相很可能普遍存在于下地幔。相关研究发表于《国家科学评论》。
