Quasi-reconstructive phase transition in ZnTe - Dr. Qingyang Hu
MARCH 4, 2020
New study from a team of scientists led by Dr. Qingyang Hu and Prof. Shengcai Zhu from Sun Yat-sen University found a quasi-reconstructive phase transition in ZnTe under different stress conditions. While phase transitions come in either displacive or reconstructive type, it occurs simultaneously in ZnTe when it was under pressure. The special type of phase transition reveals a previously undefined “grey zone” in the well-established phae transition theories. Upon the transition, ZnTe shows narrowed electronic bandgap, which provides insights for materials design and engineering in II-VI semiconductors. The study is published in the Journal of Materials Chemistry C.
Phase transitions are very common phenomenon around us—for instance, water to ice or steam. In chemistry and crystallography, there are two basic types of phase transitions. One is called displacive phase transition, which involves minor atomic movements without chemical bonds break from one phase to another. The other, called reconstructive phase transition, in which the original structure breaks significantly and rebuild into a completely different one. The latter actually constitutes the major type of phase transitions in nature. Reconstructive phase transition usually comes with large latent heat, sluggish transition hysteresis and the changes of crystal morphology.
ZnTe is an important semiconductor material for photosynthesis and other optical industry. Previous experiments showed that ZnTe goes through two displacive structural changes under compression without any destruction of the whole structure. Therefore, its chemical properties are gradually changed. This is commonly found in all group II-VI semiconductors.
Recent work led by Dr. Yukai Zhuang, a postdoc of Dr. Qingyang Hu’s group, and co-workers found a phase transition that is neither fully dispacive transition nor fully resconstructive one in compressed ZnTe using synchrotron X-ray diffraction, Raman spectroscopy, Scanning Electric Microscope (SEM) measurements and first-principles calculations. They named this mixed transition as “quasi-reconstructive” phase transition, meaning the commonly-regarded displacive phase transition in ZnTe also shares the features of a reconstructive-type when pressure is applied differently.
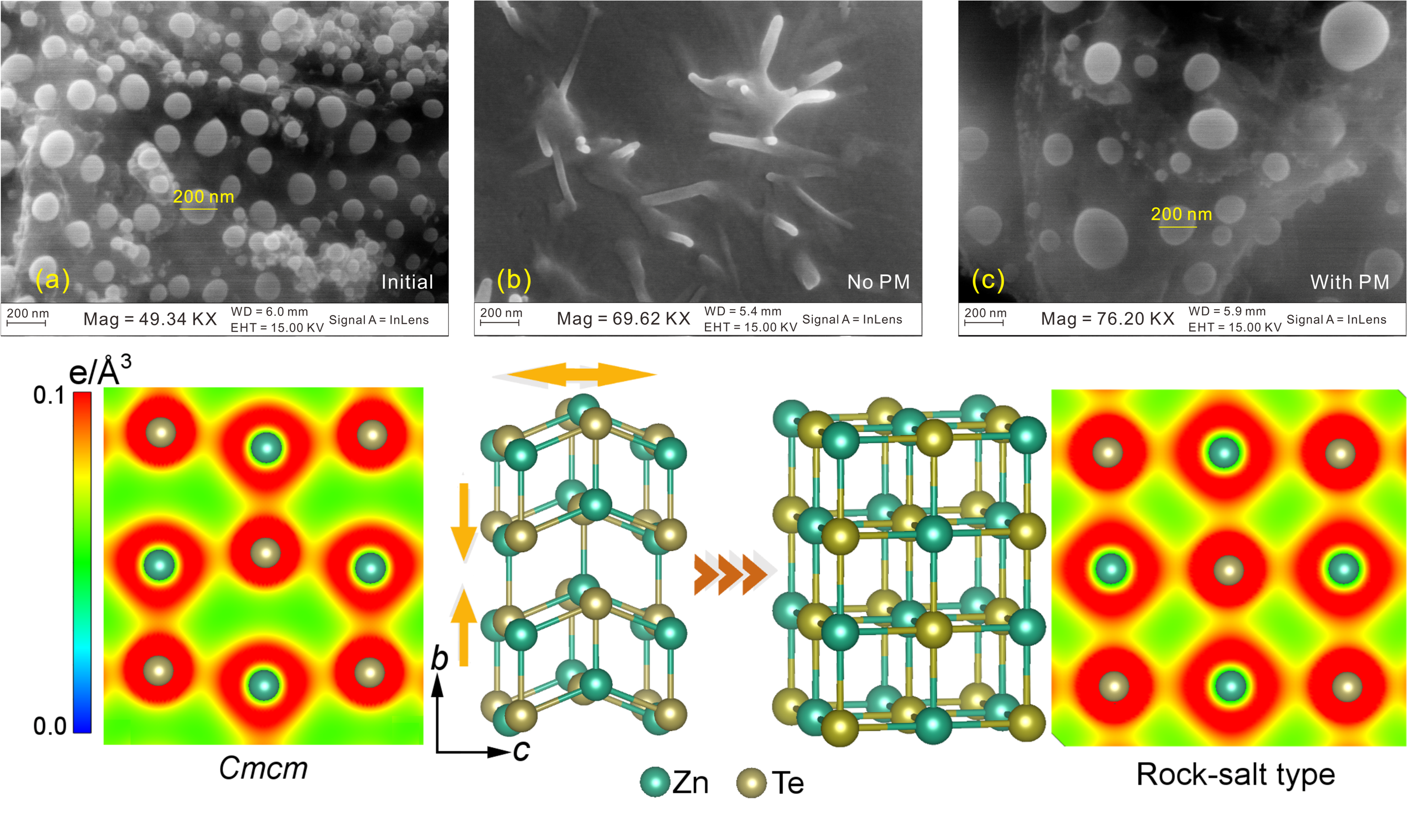
Caption: Top: (a) The SEM image of initial sample. (b) ZnTe recovered from 25.9 GPa at non-hydrostatic conditions. (c) ZnTe recovered from 25.5 GPa at quasi-hydrostatic conditions. Bottom: Charge density map showing the reconstruction of atomic bonding in ZnTe-III and rocksalt ZnTe.
In this study, the team compressed the ZnTe samples under different conditions with/without using pressure mediums. For the sample without using pressure media, ZnTe is considered to undergo large deviatoric stress. Under such condition, the phase transition point and the nature of the compressed phase have been changed. ZnTe enters a mixed phases around 15 gigapascals—the previously found ZnTe-III phase with about 15 percent of rocksalt phase. ZnTe-III came with small-range atomic displacements similar to the previously found dispacive transition while the rocksalt phase involved the formation of new chemical bonds modified by crystal morphology. These features belong to a reconstructive transition. Therefore, we define the phase transition in ZnTe under deviatoric stress a “quasi-reconstructive” type transition.
"Our study shows that deviatoric stress, although was historically regarded as “stumbling block” for material manufacturing, while it may also help engineer transition pathways and further control the transition kinetics to optimize some materials,” said Yukai.
固态相变通常指在外界压力和温度的调控下,物质晶体结构和电子结构等发生变化。在结晶学领域,根据晶体中原子位移的程度,固态相变被清晰的分为两类,一类是位移型相变,这种相变保持了初始相化学键的稳定性;另一类是重构型相变,这种相变预示着初始结构的化学键已经遭到了破坏。一般认为位移型和重构型相变之间有明显的界限。 北京高压科学研究中心的胡清扬研究员与中山大学的朱升财副教授合作的最新研究发现碲化锌(ZnTe)在差应力,也就是不均一压力的诱导下就发生了一种介于位移型和重构型间的“准重构型相变”— 该相变兼具两种相变类型的特点,处于位移型和重构型相变间的灰色地带。相关研究以“Deviatoric stress induced quasi-reconstructive phase transition in ZnTe”为题发表于J. Mater. Chem. C。
