The temperature – induced amorphous CaCO3 may help carbon cycling – Dr. Mingqiang Hou
APRIL 30, 2019
New work from a team led by HPSTAR's Dr. Mingqiang Hou confirmed that aragonite — a high-pressure mineral form of CaCO3, will turn to a stable amorphous phase at the subduction zone conditions. Their findings published in a paper in the journal Nature Communications, proposed that the amorphous calcium carbonate may help cycle carbon to the surface.
Calcium carbonate (CaCO3) plays an import role in carbon cycling, so any new discovering on it is potentially important. For its phases, there have been extensive studies. However, for the conditions of above 3 GPa and 1000K, previous experiments show controversial results about its crystal structures.
To clarify this mysterious, Hou and his colleagues set out to probe these condition transitions in CaCO3 using a pressure vessel called large-volume press combined with synchrotron x-ray diffraction. They found that the aragonite CaCO3 becomes amorphous upon heating above 3 GPa and 1000K and the amorphous state turns back to aragonite with temperature decreasing. These indicate that the transition is reversible.
"We conducted seven runs of experiments and the repeated results were consistent with the features of an amorphous phase instead of the previously reported disordered or crystallized phases”, explained Dr. Hou. “The temperature-induced amorphization in CaCO3 might be caused by the collapse of the aragonite crystal structure during heating”.
They also estimated the density of the amorphous phase from the diffraction data and found that the amorphous CaCO3 might be one of the lightest components in subduction zone. So it would likely escape from the earth interior into the surface via volcano eruption, serving as an important carbon source in the atmosphere.
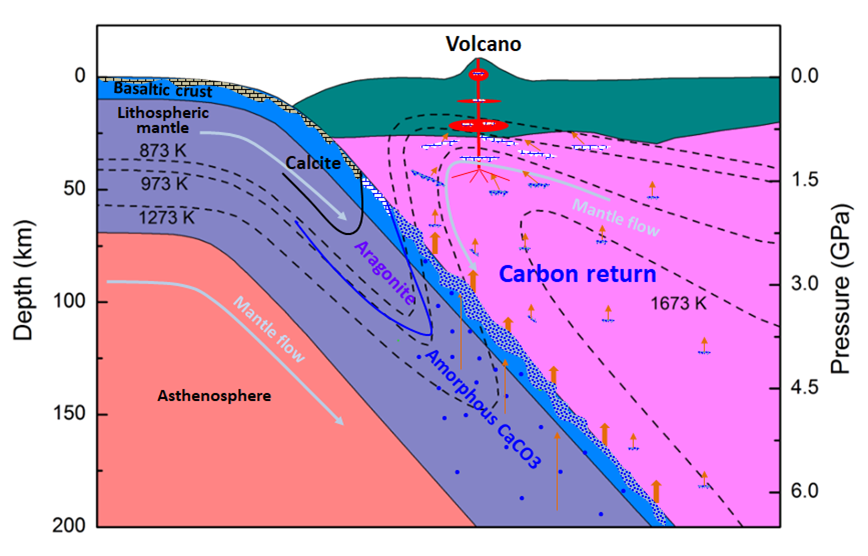
Caption: Phases of CaCO3 at upper mantle conditions and the schematic for deep carbon recycle.
碳酸盐作为含碳的重要矿物,能够通过大洋岩石圈深俯冲作用进入到地幔中,从而将地表的碳元素带到地球深部,并在火山爆发等地质活动中将地球内部的碳又带回地表。其中,碳酸钙作为一种碳酸盐,在碳的循环中扮演着尤为重要的载体角色。因此也是地球科学家们研究的重要矿物。北京高压科学研究中心的最新研究发现其文石相的碳酸钙会在3 万大气压,1000 K的以上的条件下非晶化。该研究结果澄清了关于碳酸钙在3万大气压,1000K以上的温压条件下的结构问题,并指出非晶态的碳酸钙可能是俯冲带密度最小的成份之一,因此可能有助于将地球内部的碳元素带到地表。相关研究发表于近期的Nature Communication。
