Highly reversible oxygen redox promoted by manganese site vacancy - Dr. Mingxue Tang
JULY 22, 2019
Extra capacity can be achieved by the redox of oxygen anions in addition to the redox of transition metals (Li/Na rich cathodes). However, the challenges remain in Li/Na rich cathodes are the reversible oxygen redox and the voltage decay/ hysteresis. A team of scientists co-led by Dr. Mingxue Tang from HPSTAR systematically investigated the mechanism behind the highly reversible oxygen redox in a Na-rich battery, which is published on a recent issue of Chemistry of Materials.
The fast-growing demand for safe energy storages with high power and energy density drives the continuous improvement of rechargeable Li/Na ion batteries. The large voltage hysteresis remains one of the biggest barriers to commercializing Li/Na-ion cathodes using lattice anionic redox reaction, despite their very high energy density and relative low-cost.
In this work, the team of scientists perceived a promising way to utilize lattice oxygen redox with very small voltage hysteresis for Na2Mn3O7 cathode used for sodium-ion batteries. They used EPR/NMR and XRD to reveal that a manganese site vacancy on the transition metal layer can promote reversible lattice oxygen redox reaction in the layered Na2Mn3O7.
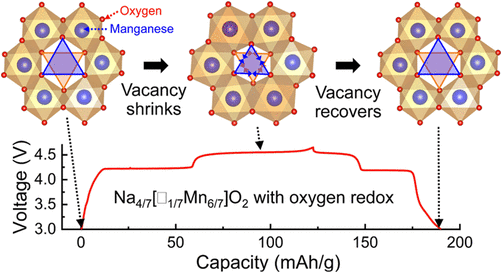
The exceptional small voltage hysteresis (< 50mV) between charge and discharge curves is mainly due to the well-maintained oxygen stacking sequence in the absence of irreversible gliding of oxygen layers and cation migration from the transition metal layers.
The shrinking of the vacant MnO6 octahedra upon charging is identified as the major driving force for the large volume decrease when Na+ is removed from the prismatic sites. The vacancy-mediated asymmetric shrunk structure is not stable and dynamically relaxes after couples of days, revealed by ex situ EPR study.
This discovery paves a new route to achieve lattice oxygen redox in layered oxide cathodes without introducing large voltage hysteresis, and hence it may inspire the exploration of new cathode materials that can achieve both high energy density and efficiency by using lattice anionic redox, with minimum voltage decay.
Caption: Understanding the Low-Votage Hystersis of Anionic Redox in Na2Mn3O7.
利用晶格阴离子氧化还原反应设计的Li / Na离子电池的正极材料,具有高能量密度和价格低廉的优势。本工作借助电子顺磁共振EPR、核磁共振NMR、以及X射线衍射XRD对正极材料Na2Mn3O7的充放电机理进行系统研究。揭示了在充放电曲线中出现的低电压滞后的原因是由于缺乏不可逆的氧层滑移和过渡金属层阳离子迁移,氧原子能很好地保持堆叠顺序。开始充电时,Na2Mn3O7体积变化非常小(低于0.1%),进一步充电导致空缺的MnO6八面体的收缩,驱动六面棱柱位置Na+的脱嵌而导致体积减小。这一发现为研究层状氧化物正极材料中晶格作为氧化还原中心开辟了一条新的途径,并且不会产生大电压滞后作用。从而有助于探索晶格阴离子的可逆氧化还原反应,进一步获得高能量密度、高效的新型正极材料。
