Chemistry Design Towards a Stable Sulfide-based Superionic Conductor Li4Cu8Ge3S12 - Drs. Yingqi Wang, Xujie Lü and Wenge Yang
APRIL 17, 2019
Sulfide-based superionic conductors with high ionic conductivity have been explored as a promising candidate for solid-state Li batteries. However, their hypersensitive nature against moisture has not only made their manufacturing process complicated and costly, but also impeded their applications in novel batteries including the aqueous Li-air and Li-redox-flow designs. A sulfide-based ionic conductor Li4Cu8Ge3S12 with superior stability was developed by Drs. Yingqi Wang, Xujie Lü and Wenge Yang at HPSTAR, in collaboration with Prof. Fuqiang Huang at Peking University. The material features a rigid Cu-Ge-S open framework that increases its stability. Meanwhile, the weak bonding between Li+ and the framework promotes ionic conductivity. This study is published in Angewandte Chemie International Edition.
Lithium-ion batteries (LIBs) are widely used in portable electronic devices and electric vehicles. The exploration of superior solid electrolytes to realize all-solid-state LIBs is currently under the spotlight and could address safety issues, render a higher electrochemical window, and fit better for flexible devices. Sulfide-based superionic conductors with high ionic conductivity have been explored as a promising candidate for solid-state Li batteries. However, their hypersensitive nature against moisture has not only made their manufacturing process complicated and costly but also impeded their applications in novel batteries including the aqueous Li-air and Li-redox-flow designs. Development of new compounds with a designed structure for higher chemical stability is, therefore, highly desirable.
Here a group of HPSTAR scientists with collaborators from Peking University and Argonne National Laboratory reported a new sulfide-based superionic conductor Li4Cu8Ge3S12 with superior stability and high ionic conductivity. A Cu-Ge-S anionic open framework provides a rigid and stable scaffold. Meanwhile, the weak bonding between Li+ and the framework promotes ionic conductivity. The as-designed compound is stable in both moist air and LiOH aqueous solution. An ionic conductivity of 0.9×10-4 S/cm with a low activation energy of 0.33 eV has been achieved without any optimization.
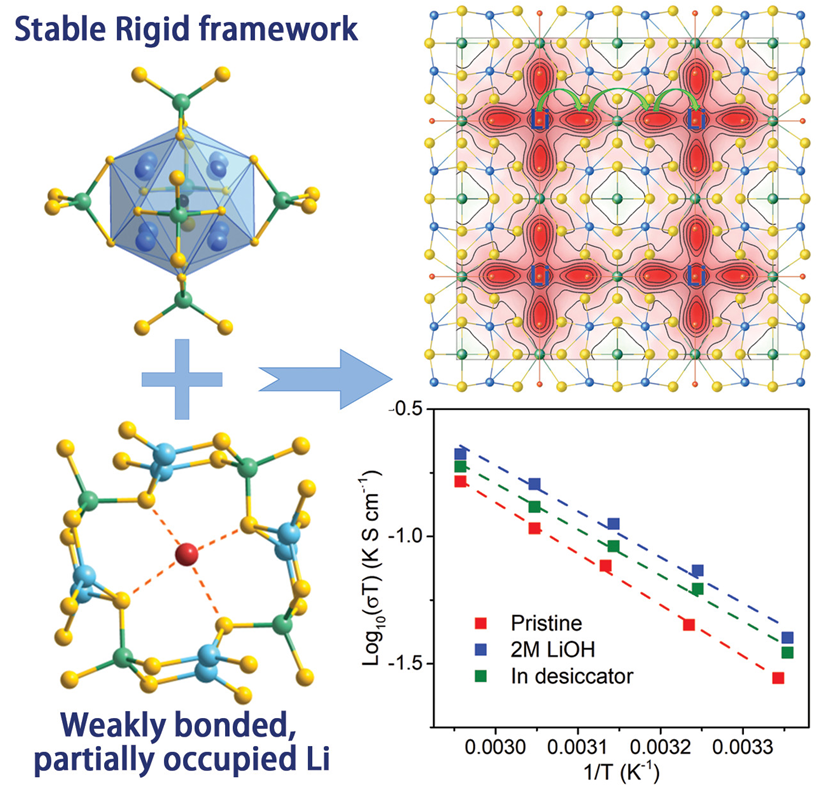
“Our work provides a unique chemical and structural configuration where the weakly-bonded Li in a rigid framework endues both high conductivity and outstanding stability, which could inspire scientists to develop more advanced solid electrolytes.” said Dr. Xujie Lü, one of the corresponding authors.
硫族化合物是固态电解质家族中的重要一类,近些年发现的许多硫化物固态电解质具有优异的离子传输性能,但通常对水和空气不稳定。北京高压科学研究中心的王瑛琪、吕旭杰和杨文革博士与北京大学黄富强教授等合作,通过巧妙的设计获得了一种同时具有优异稳定性和良好Li+电导率的硫化物锂离子导体,Li4Cu8Ge3S12。刚性Cu-Ge-S阴离子框架提供了稳定的支架,Li+位于框架内部通道中,与框架阴离子具有弱的键合作用。该研究对于筛选和设计更多样化的固态锂电解质结构具有重要的指导意义。
