Direct Reaction between Copper and Nitrogen at HP and HT– Jack Binns
MARCH 6, 2019
Compounds of the transition metals and nitrogen have applications in many areas from ultrahard materials to explosives. To date none of the group 11 elements: copper, silver, or gold are known to form a nitride compound, MN2 despite many decades of attempts. A new study by RTH Lab of HPSTAR reports that by laser heating mixtures of the elements in diamond-anvil cells at high pressures, a direct reaction between copper and nitrogen is observed forming the new compound copper diazenide (CuN2). This study is published in The Journal of Physical Chemistry Letters.
Many of the transition metals react at high-temperatures and pressures to form nitrides which adopt many different structures ranging from ultrahard N2 ions to polymeric chains of single-bonded nitrogen. Looking across the periodic table there is a gap at group 11 where the only known nitrogen compounds are highly unstable and explosive azides, M(N3).
“Combining high pressures and temperatures has successfully produced new nitrogen compounds for other metals,” said Dr Jack Binns, the lead author of the study. “We attempted to answer the questions, could this technique create a new compound between these unfavourable reactants?”
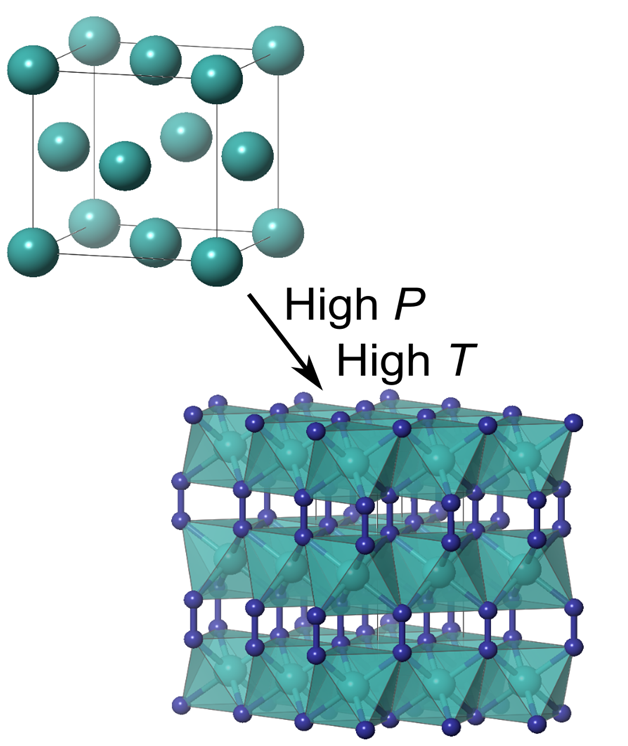
Laser heating copper in a nitrogen atmosphere creates CuN2, containing linear N2 ions linking Cu atoms together. |
Here a group of HPSTAR scientists including Jack Binns, Mary-Ellen Donnelly, Mengnan Wang, Eugene Gregoryanz, Philip Dalladay-Simpson and Ross T. Howie, in collaboration with Miriam Peña-Alvarez of The University of Edinburgh, use a combination of high pressures and temperatures to directly react copper and nitrogen. Supporting theoretical work was performed by Andreas Hermann of The University of Edinburgh.
Laser heating copper in a nitrogen atmosphere creates CuN2, containing linear N2 ions linking Cu atoms together.
The team found that heating a sample of copper above 1500 K and 50 GPa initiated a reaction forming an entirely new compound. A combination of X-ray diffraction and DFT calculations confirmed the structure and stoichiometry, CuN2, and investigated the electronic properties.
“The electronic structure of CuN2 turned out to be surprisingly complex”, said Dr. Ross Howie, the corresponding author. “The partially-filled orbitals and weak Cu-N bonds show an ambiguous oxidation state between Cu+ and Cu2+ - which hasn’t been observed in other nitrides before.”
As pressure is released, CuN2 gradually decomposes back into the elements. This work suggests that the long sought after silver and gold nitride compounds could be found at similar reaction conditions.
过渡金属氮化物—氮与过渡金属形成的氮化物,具有高熔点、高化学稳定性、耐腐蚀性以及高硬度等特点,并有导电性,它们大多是氮原子填充在金属结构的间隙中,属于“间隙化合物”, 具有金属的性质。近年来高温高压技术被广泛用来合成金属氮化物,比如TiN,AlN。北京高压科学研究中心的RHT研究小组使用金刚石压砧中的激光加热技术,在50 GPa, 1500K的条件下首次合成了铜的氮化物。借助理论计算及晶体结构拟合,他们确定生成的化合物是具有六方结构的CuN2。进一步的电子结构计算得出该CuN2具有金属的导电性以及与其他过渡金属氮化物不同的复杂的电子结构。