Oxygen valence state varies at Earth's deep mantle - Drs. Jin Liu, Qingyang Hu & Ho-kwang Mao
JANUARY 15, 2019
It is conventionally accepted that the oxygen anion has an unvarying −2 valence state for major lower mantle compositions like ferropericlase and bridgmanite. A recent study led by Dr. Ho-kwang Mao from HPSTAR found variation in the valence state of oxygen in the newly discovered Fe-O-H system under deep mantle conditions. The new finding was reported Nature Communications in January, 2019.
The redox states of iron oxides in solid Earth are mostly controlled by the 3d transition element Fe and its valence state commonly varies among metallic Fe0, ferrous Fe2+, and ferric Fe3+. Generally, oxygen is expected to remain -2 valence state in all mantle minerals. However, this convention thinking may be overturned with the emergence of FeO2Hx in the deep mantle.
“If we take oxygen as the common O2- state as in other iron oxides, the valence state of iron would be ferryl (Fe4+) for FeO2. On the other hand, based on the analogy to FeS2 pyrite wherein iron remains ferrous (Fe2+) with forming sulfur dimer, would FeO2 also consist of Fe2+ cation and oxygen dimer?” said Dr. Mao. “As FeO2 is able to hold a variable amount of hydrogen to form FeO2Hx (0≤x≤1), what type of bonding between Fe and O? Then what is the valence state of hydrogen? Thus far, theoretical calculations cannot provide a conclusive answer.”
The HPSTAR team also includes Drs. Qingyang Hu, Liuxiang Yang, who carried out challenging experiments by combining a battery of state-of-the-art synchrotron x-ray spectroscopic techniques supplemented with first-principles simulation to shed light on the puzzling chemistry state of pyrite-type FeO2Hx.
They found that despite the high O/Fe ratio in FeO2Hx, iron remains in the ferrous, spin-paired and non-magnetic state at 60–133 GPa while the presence of hydrogen has minimal effects on the valence of iron. The reduced iron is accompanied by oxidized oxygen due to oxygen-oxygen interaction. Unlike the fixed -2 state of oxygen in all other major mantle minerals, it varies around –1 in FeO2Hx. This result indicates that like Fe, O may have multiple valence states in our planet’s interior. Our study suggests a possible change in the chemical paradigm of how O, Fe and H behave under deep Earth conditions.
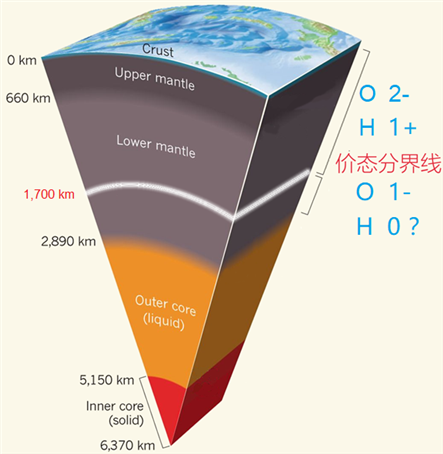
Caption: The oxygen and hydrogen cycling in the deep Earth.
一直以来氧元素以-2价存在于地球矿物中。然而由北京高压科学研究中心的胡清扬研究员带领的国际小组的最新研究发现氧元素在新发现的下地幔主要成份—FeO2Hx中并不是传统的-2价,而表现出新的化合价态。另地球内部的的主要挥发性元素氢在地球深部亦可产生新的稳定价态。这项工作表明在下地幔距离底表1700-1900千米处可能存在一个价态分界层,地球内部的主要元素铁和氧以及挥发性元素氢在分界层以下会呈现全新的化学性质。这一结果于2019年1月发表在《自然·通讯》,而后经由理论计算验证,推测了新价态的产生机制,计算结果同期发表于《无机化学》。
