Publications
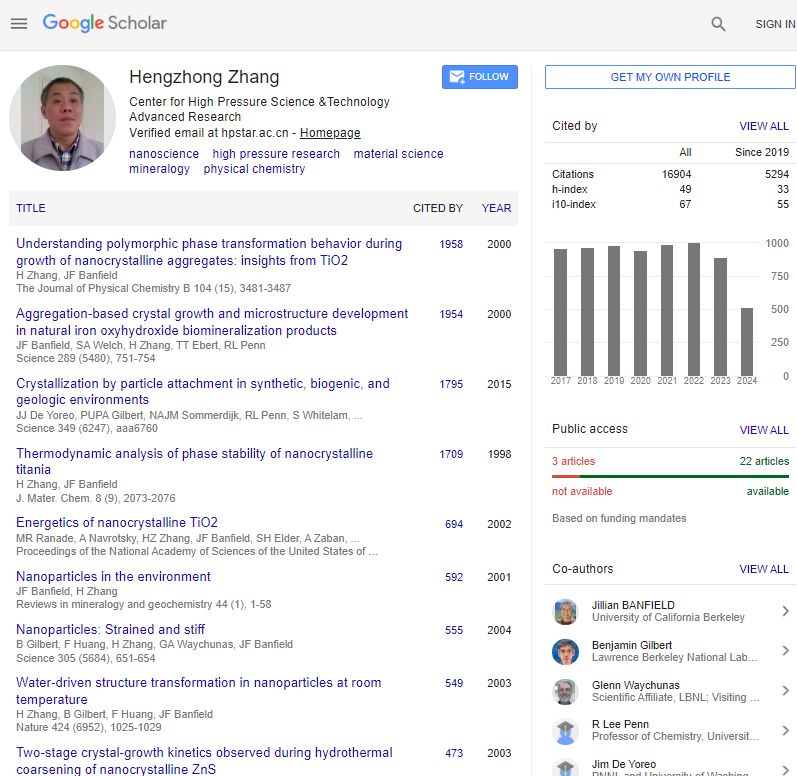
Selected publications (out of 100+; Google Scholar citations > 16,000; h-index 49):
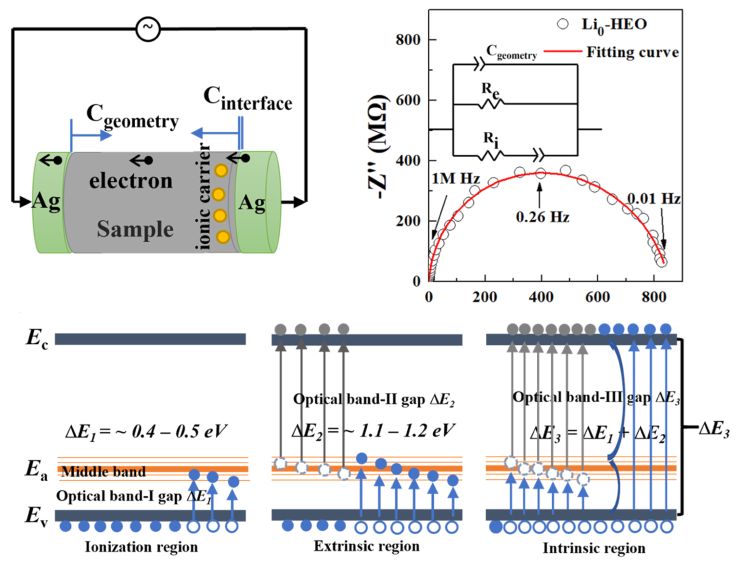
Song, M.; Zhang, X.; Wan, S.; Wang, G.; Liu, J.; Li, W.; Dong, H.; Lou, C.; Chen, Z.; Chen, B.; Zhang, H.* Electrical Conductivities and Conduction Mechanism of Lithium-Doped High-Entropy Oxides at Different Temperature and Pressure Conditions. JACS Au 2024, 4 (2), 592–606.
微信公众号简报
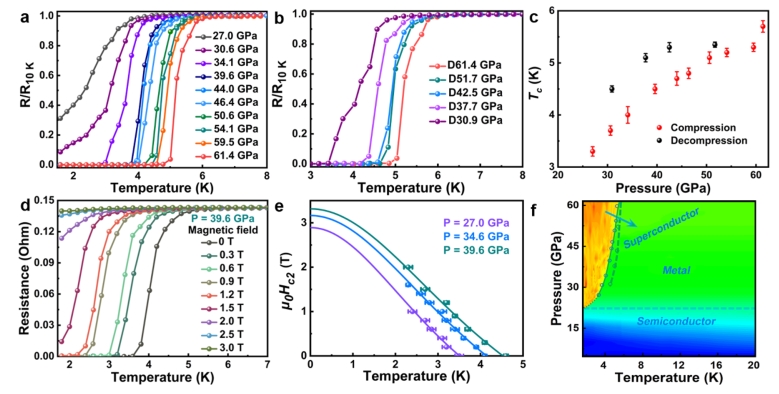
Zhang, X.; Li, W.; Feng, J.; Song, M.; Wang, G.; Liu, J.; Wang, Z.; Jiang, S.; Sheng, H.; Chen, B.; Zhang, H.* Pressure-Induced Structural and Semiconductor-Metal-Superconductor Transitions in a High-Entropy van Der Waals Compound (MnFeCuCdIn)PSe3. Adv. Quantum Technol. 2024, 2300365.
微信公众号简报
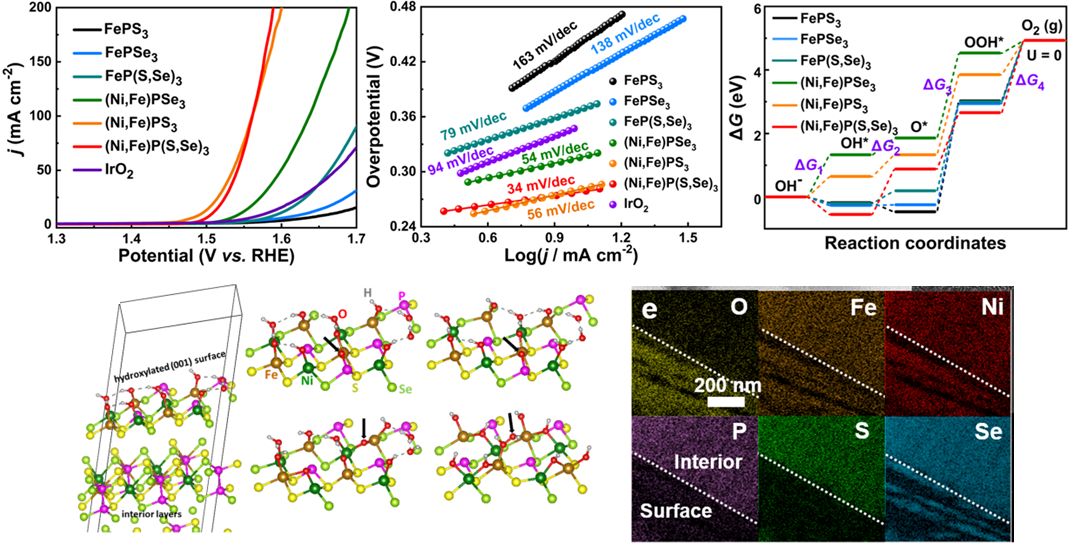
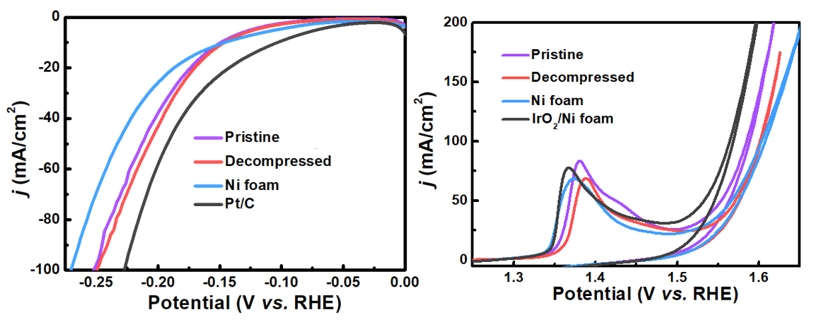
Li, W.; Li, C.; Dong, H.; Zhang, X.; Liu, J.; Song, M.; Wang, G.; Zhao, L.; Sheng, H.; Chen, B.; Zhang, H.* Expediting Oxygen Evolution by Optimizing Cation and Anion Complexity in Electrocatalysts Based on Metal Phosphorous Trichalcogenides. Angew. Chem. Inter. Ed. 2023, 62, e202214570.
微信公众号简报
Zhang, X.; Li, W.; Wan, S.; Feng, J.; Song, M.; Liu, J.; Wang, G.; Chen, Z.; Chen, B.; Zhang, H.* Pressure Dependence of the Electrical Conductivities of High-Entropy Diborides. J. Euro. Ceram. Soc. 2022, 42 (15), 6951–6957.
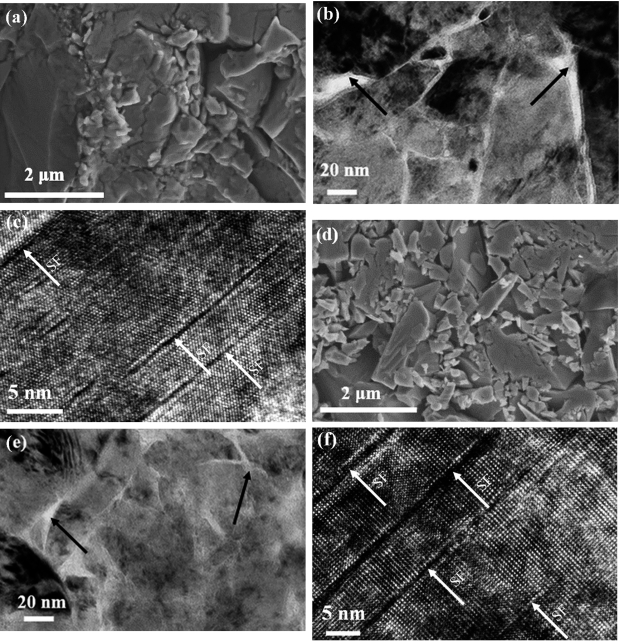
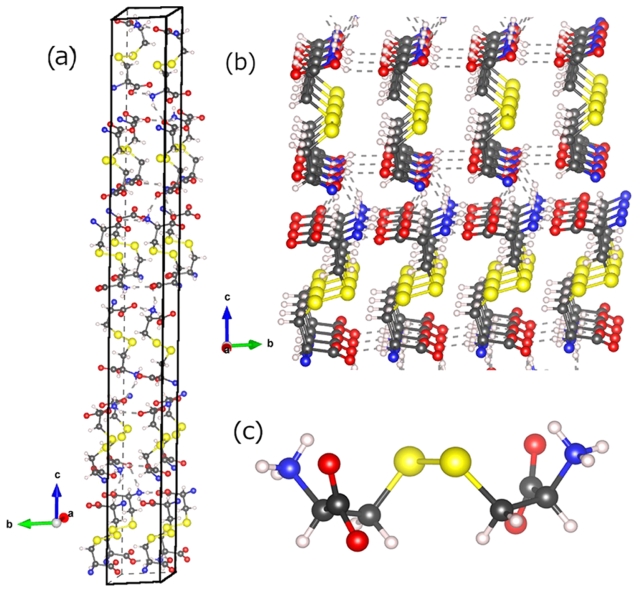
Li, W.; Feng, J.; Zhang, X.; Li, C.; Dong, H.; Deng, W.; Liu, J.; Tian, H.; Chen, J.; Jiang, S.; Sheng, H.; Chen, B.; Zhang, H.*Metallization and Superconductivity in the van der Waals Compound CuP2Se through Pressure-Tuning of the Interlayer Coupling. J. Am. Chem. Soc. 2021, 143, 20343–20355.
微信公众号简报1; 微信公众号简报2
Yan, J.; Zhang, L.; Liu, J.; Li, N.; Tamura, N.; Chen, B.; Lin, Y.; Mao, W. L.; Zhang, H.Pressure-Induced Suppression of Jahn–Teller Distortions and Enhanced Electronic Properties in High-Entropy Oxide (Mg0.2Ni0.2Co0.2Zn0.2Cu0.2)O. Appl. Phys. Lett. 2021, 119, 151901.
Song, M.; Li, W.; Zhang, X.; Liu, J., Li, K.; Zhang, H.* Structural Stability of L-Cystine under Extreme Conditions. ACS Earth Space Chem., 2021, 5, 1525 - 1534.
Zhang, X.; Li, W.; Tian, H.; Liu, J.; Li, C.; Dong, H.; Chen, J.; Song, M.; Chen, B.; Sheng, H.; Wang, S.; Zhang, D.; Zhang, H.*Ultra-Incompressible High-Entropy Diborides. J. Phys. Chem. Lett. 2021, 3106–3113.
微信公众号简报
Liu, J.; Chen, J.; Li, W.; Tian, H.; Zhang, X.; Li, N.; Yan, J.; Kunz, M.; Chen, B.; Zhang, H.* Differentiating Electrical and Optoelectrical Properties of Oxysulfides La2Ta2MS2O8 (M=Zr, Ti) via Application of Pressure. J. Phys. Chem. C 2020, 124, 14477 - 14484.
Zhou, X.; Feng, Z.; Zhu, L.; Xu, J.; Miyagi, L.; Dong, H.; Sheng, H.; Wang, Y.; Li, Q.; Ma, Y.; Zhang, H.; Yan, J.; Tamura, N.; Kunz, M.; Lutker, K.; Huang, T.; Hughes, D. A.; Huang, X.*; Chen, B.* High-Pressure Strengthening in Ultrafine-Grained Metals. Nature 2020, 579 (7797), 67–72.
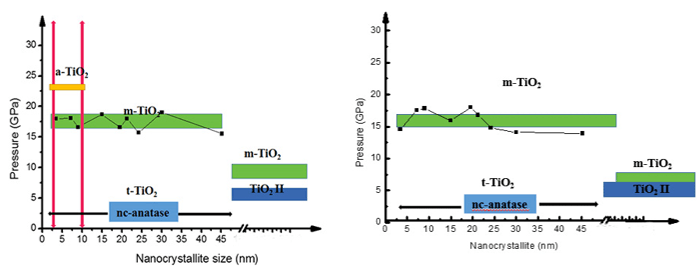
Zhang, X.; Tian, H.; Li, W.; Liu, W.; Chen, J.; Liu, J.; Han, X.; Yan, B.; Chen, Z.; Gou, H.; Li, K.; Jiang, H.; Zhang, D.; Kunz, M.; Zhang, H.* High-Pressure Phase Transitions in Densely Packed Nano-Crystallites of TiO2-II. J. Phys. Chem. C 2019, 124(1), 1197-1206.
Jiang, X.; Yan, Z.; Zhang, J.; Gao, J.; Huang, W.*; Shi, Q.*; Zhang, H. Mesoporous Hollow Black TiO2 with Controlled Lattice Disorder Degrees for Highly Efficient Visible-Light-Driven Photocatalysis. RSC Adv. 2019, 9 (63), 36907–36914.
Chen, B.*; Huang, Y.; Xu, J.; Zhou, X.; Chen, Z.; Zhang, H.; Zhang, J.; Qi, J.; Lu, T.; Banfield, J. F.; et al. Revealing the Ductility of Nanoceramic MgAl2O4. J. Mater. Res. 2019, 34 (9), 1489–1498
Chen, J.; Liu, W.; Liu, J.; Zhang, X.; Yuan, M.; Zhao, Y.; Yan, J.; Hou, M.; Yan, J.; Kunz, M.; Tamura, N.; Zhang, H.*; Yin, Z.* Stability and Compressibility of Cation-Doped High-Entropy Oxide MgCoNiCuZnO5, J. Phys. Chem. C 2019, 123(9), 17735-17744.
Liu, J.; Yan, J.; Shi, Q.; Dong, H.; Zhang, J.; Wang, Z.; Huang, W.; Chen, B.; Zhang, H.*Pressure Dependence of Electrical Conductivity of Black Titania Hydrogenated at Different Temperatures. J. Phys. Chem. C 2019, 123 (7), 4094–4102.
Liu, W.; Chen, J.; Zhang, X.; Yan, J.; Hou, M.; Kunz, M.; Zhang, D.; Zhang, H.* Pressure-Induced Phase Transitions of Natural Brookite. ACS Earth Space Chem. 2019, 3 (5), 844–853.
Ke, F.; Chen, Y.; Yin, K.; Yan, J.; Zhang, H.; Liu, Z.; Tse, J. S.; Wu, J.; Mao, H.*; Chen, B.* Large Bandgap of Pressurized Trilayer Graphene. PNAS, 2019, 116 (19), 9186-9190.
Chen, B.*; Lin, J.-F.; Chen, J.; Zhang, H.; Zeng, Q. Synchrotron-Based High-Pressure Research in Materials Science. MRS Bulletin 2016, 41 (06), 473–478.
Liu, G.*; Kong, L.; Yan, J.; Liu, Z.; Zhang, H.; Lei, P.; Xu, T.; Mao, H.; Chen, B.*Nanocrystals in Compression: Unexpected Structural Phase Transition and Amorphization Due to Surface Impurities. Nanoscale 2016, 8 (23), 11803–11809.
De Yoreo, J. J.; Gilbert, P. U. P. A.; Sommerdijk, N. A. J. M.; Penn, R. L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J. D.; Navrotsky, A.; Banfield, J. F.; Wallace, A. F.; Michel, F. M.; Meldrum, F. C.; Colfen, H.; Dove, P. M.*Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. DOI: 10.1126/science.aaa6760.
Zhang, H.*; Banfield, J. F.Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2.Chem. Revs. 2014, 114, 9613 – 9644 .
Zhang, H.; De Yoreo, J. J.; Banfield, J. F.* A unified description of attachment-based crystal growth. ACS Nano, 2014, 8, 6526 – 6530.
Zhang, H.*; Banfield, J. F.Interatomic Coulombic interactions as the driving force for oriented attachment.CrystEngComm, 2014, 16, 1568-1578.
Zhang, H.*; Finnegan, M. F.; Banfield, J. F. Titania nanorods curve to lower their energy.Nanoscale 2013, 5, 6742 – 6746.
Zhang, H.*; Banfield, J.F.* Energy calculations predict nanoparticle attachment orientations and asymmetric crystal formation.J. Phys. Chem. Lett. 2012, 3, 2882-2886.
Zhuang, Z,; Huang, F.; Lin, Z*.; Zhang, H.*Aggregation-induced fast crystal growth of SnO2 nanocrystals.J. Am. Chem. Soc. 2012, 134, 16228-16234.
Fernando, S; Baynes, M; Chen, B; Banfield, J. F.; Zhang, H.*Compressibility and structural stability of nanoparticulate goethite. RSC Adv. 2012, 2, 6768 -6772.
Zhang, H.*; Chen, B.; Banfield, J. F. The size dependence of the surface free energy of titania nanocrystals. Phys. Chem. Chem. Phys. 2009, 11, 2553.
Chen, B.*; Zhang, H.; Dunphy-Guzman, K. A.; Spagnoli, D.; Kruger, M. B.; Muthu, D. V. S.; Kunz, M.; Fakra, Sirine; Hu, J. Z.; Guo, Q. Z.; Banfield, J. F. Size-dependent elasticity of nanocrystalline titania.Phys. Rev. B 2009, 79, 125406.
Zhang, H.*; Chen, B.; Waychunas, G. A.; Banfield, J. F.Atomic structure of nanometer-sized amorphous TiO2. Phys. Rev. B 2008, 78, 214106.
Chen, B.*; Zhang, H.; Gilbert, B.; Banfield, J. F.Mechanism of inhibition of nanoparticle growth and phase transformation by surface impurities.Phys. Rev. Lett. 2007, 98, 106103.
Gilbert, B.; Huang, F.; Zhang, H.; Waychunas, G. A.; Banfield, J. F.* Nanoparticles: strained and stiff. Science 2004, 305, 651.
Zhang, H.*; Banfield, J. F. Aggregation, coarsening, and phase transformation in ZnS nanoparticles studied by molecular dynamics simulations.Nano Lett. 2004, 4, 713.
Zhang, H.; Gilbert, B.; Huang, F.; Banfield, J. F.*Water-driven structure transformation in nanoparticles at room temperature.Nature 2003, 424, 1025.
Zhang, H.*; Huang, F.; Gilbert, B.; Banfield, J. F. Molecular dynamics simulations, thermodynamics analysis and experimental study of phase stability of zinc sulfide nanoparticles.J. Phys. Chem. B 2003, 107, 13051.
Zhang, H.*; Finnegan, M.; Banfield, J. F. Preparing single-phase nanocrystalline anatase from amorphous titania with particle sizes tailored by temperature. Nano Lett. 2001, 1, 81.
Banfield, J. F.*; Welch, S. A.; Zhang, H.; Ebert, T. T.; Penn, R. L. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products.Science 2000, 289, 751.
Zhang, H.*; Banfield, J. F. , Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J. Phys. Chem. B 2000, 104, 3481.
Zhang, H.*; Banfield, J. F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation.J. Mater. Res. 2000, 15, 437.
Zhang, H.*; Banfield, J. F. New kinetic model for the nanocrystalline anatase-to-rutile transformation revealing rate dependence on number of particles.Am. Mineral. 1999, 84, 528.
Zhang, H.*; Banfield, J. F.Thermodynamic analysis of phase stability of nanocrystalline titania.J. Mater. Chem. 1998, 8, 2073.
Google Scholar page:
https://scholar.google.com/citations?user=87T1wFIAAAAJ&hl=en&oi=ao
ResearchGate page:
http://www.researchgate.net/profile/Hengzhong_Zhang
Orcid:
https://orcid.org/0000-0003-2322-2274
Last update July, 2024